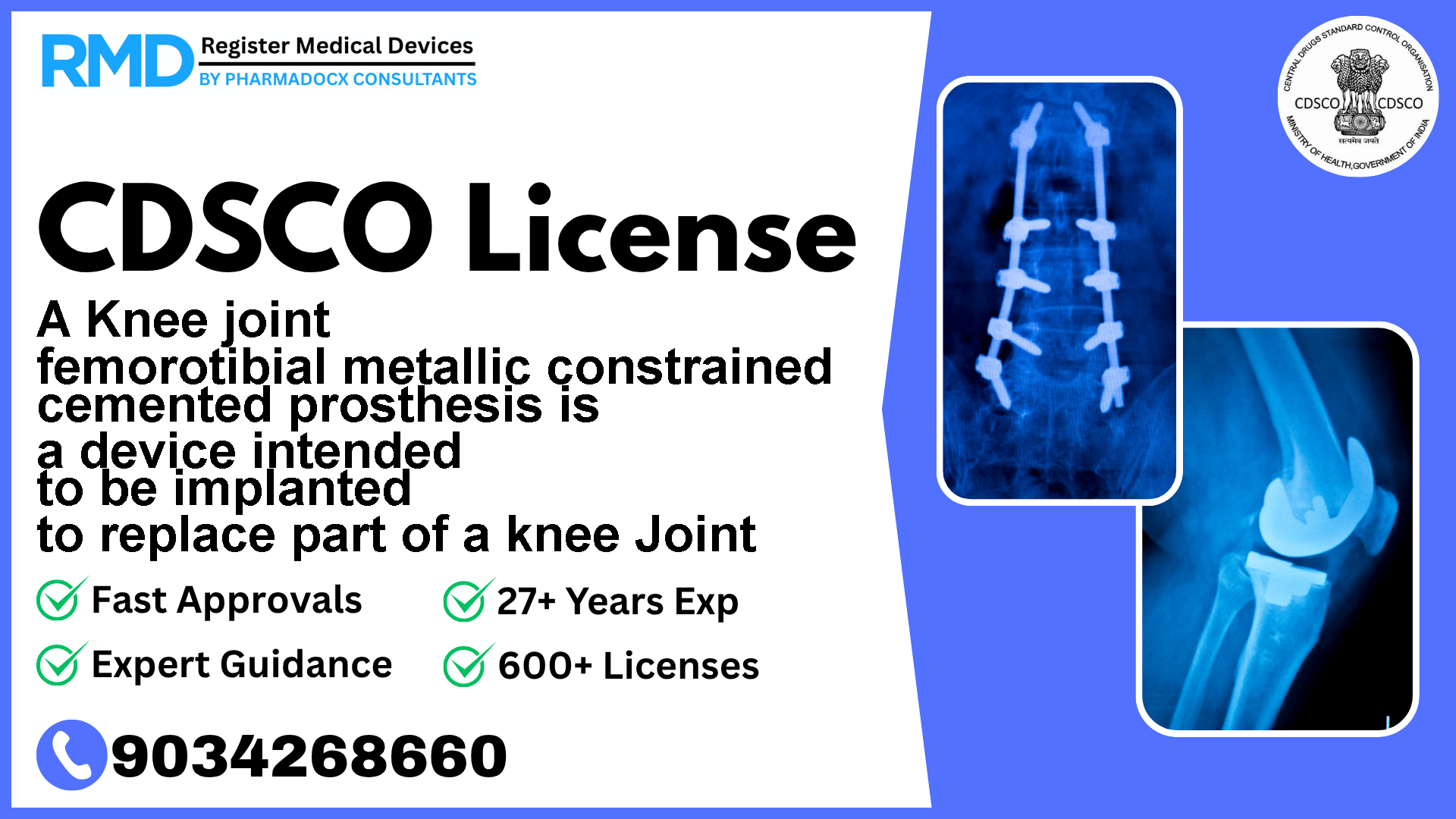
CDSCO License for A Knee joint femorotibial metallic constrained cemented prosthesis is a device intended to be implanted to replace part of a knee Joint
Medical Device Information
Intended Use
Intended to be implanted to replace part of a knee joint
Comprehensive Guide to CDSCO Licensing for Knee Joint Femorotibial Metallic Constrained Cemented Prosthesis (Class C Orthopaedic Implant)
Navigating the regulatory landscape in India for medical devices can be complex, especially for high-risk implants like the Knee Joint Femorotibial Metallic Constrained Cemented Prosthesis. With over 25 years of experience and having assisted 500+ companies in securing CDSCO licenses, we provide you with a detailed, step-by-step guide to obtain your manufacturing or import license for this Class C orthopaedic implant.
Understanding Your Device and Its Regulatory Significance
This prosthesis is a critical implantable device intended to replace part of the knee joint, specifically the femorotibial compartment. Given its direct impact on patient mobility and safety, the Central Drugs Standard Control Organization (CDSCO) classifies it as Class C — indicating a moderate to high risk.
Orthopaedic implants like this require strict regulatory compliance to ensure safety, efficacy, and quality. The CDSCO's regulatory framework mandates comprehensive documentation, testing, and audits before granting manufacturing or import licenses.
CDSCO Regulatory Framework for Orthopaedic Implants (Class C Devices)
Under the Medical Device Rules 2017, devices are classified from Class A (low risk) to Class D (high risk). For your implant, which falls under Class C, the licensing authority is the Central Licensing Authority (CLA) of CDSCO.
The relevant license for manufacturing is the MD9 license (Application Form MD7), and for import, it is the MD15 license (Application Form MD14). The notified device falls under Notification No. 29/Misc/3/2017-DC (292), dated 06.06.2018, which specifies regulatory requirements for orthopaedic implants.
Risk Classification and License Requirements for Your Device
| Device Name | Risk Class | License Type | Licensing Authority |
|---|---|---|---|
| Knee Joint Femorotibial Metallic Constrained Cemented Prosthesis | C | MD9 | Central Licensing Authority |
This classification mandates rigorous testing, documentation, and audits before license issuance.
Manufacturing License Process (MD9) for Class C Devices
Test License Acquisition (Form MD13):
- Duration: Approximately 1.5 to 2 months.
- Purpose: To enable initial product testing in CDSCO-approved labs.
Product Testing:
- Conducted at government-approved testing laboratories.
- Tests cover biocompatibility, mechanical strength, sterilization validation, and more.
- Refer to the list of approved testing laboratories for selection.
Document Preparation:
- Compilation of technical files, Device Master File, Plant Master File, Essential Principles checklist, Risk Management File, Quality Management System (QMS) documentation, and more.
Application Submission:
- Apply online via the CDSCO MD Online Portal using Form MD7.
Inspection and Audit:
- CDSCO inspectors will audit manufacturing facilities for compliance.
- Ensure readiness by following guidelines in our MD9 License guide.
Query Resolution:
- Address any queries raised by CDSCO promptly.
Grant of License:
- Upon successful audit and documentation review, the MD9 license is granted.
Manufacturing License Documents Required for MD9
- Company Constitution: Certificate of Incorporation, Partnership Deed, or Proprietorship documents.
- Proof of Premises Ownership or Lease: Valid ownership documents or lease agreements.
- Technical Staff Details: Qualification and experience certificates for key personnel.
- Fire NOC & Pollution Control Certificate: Compliance with local statutory requirements.
- Device Master File (DMF): Detailed product description, manufacturing process, and design validation. Our Device Master File guide provides comprehensive insights.
- Plant Master File (PMF): Details of manufacturing facilities, equipment, and processes. Learn how to prepare a PMF here.
- Essential Principles Checklist: Compliance with Indian Essential Principles for Medical Devices.
- Risk Management File: Documented hazard analysis and risk mitigation following ISO 14971. See our Risk Management guide.
- Test Reports: From CDSCO-approved labs validating product safety and performance.
- Labels and Instructions for Use (IFU): As per regulatory norms.
- Quality Management System Documents: ISO 13485:2016 certification and internal QMS procedures.
Import License Process (MD15) for Class C Devices
Manufacturers or importers aiming to bring this device into India need to obtain an MD15 import license issued by the Central Licensing Authority.
Document Preparation:
- Collate all required documents, including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate (if applicable), DMF, PMF, Wholesale License, and Company Constitution.
Application Submission:
- File Form MD14 on the CDSCO MD Online Portal.
Review and Queries:
- Respond promptly to any CDSCO queries.
Grant of License:
- The import license is granted typically within 5-6 months.
Import License Documents Required
- Valid Manufacturing License from the country of origin.
- Free Sale Certificate or equivalent.
- ISO 13485:2016 QMS Certificate.
- CE Certificate (if device is CE marked).
- Device Master File and Plant Master File.
- Wholesale License for import/distribution.
- Company Constitution documents.
Timeline and Processing Duration
| License Type | Typical Duration |
|---|---|
| MD13 Test License | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| MD9 Manufacturing License | 4 - 5 months (including above) |
| MD15 Import License | 5 - 6 months |
Planning your regulatory strategy accordingly can prevent costly delays.
Government Fees and Costs
MD9 Manufacturing License:
- Application Fee: ₹50,000
- Per Product Fee: ₹1,000
MD13 Test License:
- Approximately ₹5,000 to ₹10,000 (varies by state)
MD15 Import License:
- For Class C devices: USD 3,000 per site + USD 1,500 per product
Note: All fees are payable online during application submission on the CDSCO portal.
Common Challenges and Practical Solutions
Challenge 1: Delays in Product Testing
- Solution: Choose CDSCO-approved labs with proven turnaround times. Early engagement accelerates testing.
Challenge 2: Incomplete Documentation
- Solution: Use detailed checklists and templates for DMF, PMF, and Risk Management files. Our guides linked above offer valuable templates.
Challenge 3: Non-compliance During Audits
- Solution: Conduct thorough internal audits before CDSCO inspections. Engage notified bodies and consultants for mock audits.
Challenge 4: Query Resolution Delays
- Solution: Assign a dedicated regulatory liaison to respond quickly to CDSCO queries.
Expert Consultation and Support
Given the complexity and high stakes involved, partnering with experienced regulatory consultants can streamline your license acquisition. Our team has successfully guided 500+ companies through the CDSCO process for orthopaedic implants, ensuring compliance and timely approvals.
We offer:
- End-to-end documentation support
- Application filing and follow-up
- Internal audits and gap analysis
- Training for your technical staff
Getting Started with Your CDSCO License Application
Assess your device classification: Confirm your device is Class C via the Medical Device Classification resource.
Prepare your test license application (Form MD13): Begin with this to initiate product testing.
Select an approved testing laboratory: Use the official Testing Laboratories list.
Compile required documentation: Leverage our Device and Plant Master File guides.
Submit your applications through the CDSCO MD Online Portal: Ensure all forms and fees are accurately completed.
Plan for audits and inspections: Coordinate with CDSCO and notified bodies well in advance.
Maintain continuous communication: Promptly address any queries from CDSCO.
By following these steps and leveraging expert guidance, you can efficiently obtain your CDSCO manufacturing or import license for the Knee Joint Femorotibial Metallic Constrained Cemented Prosthesis, positioning your product for successful entry into the Indian market.
For detailed, personalized support, contact our regulatory experts today and ensure your compliance journey is smooth and successful.