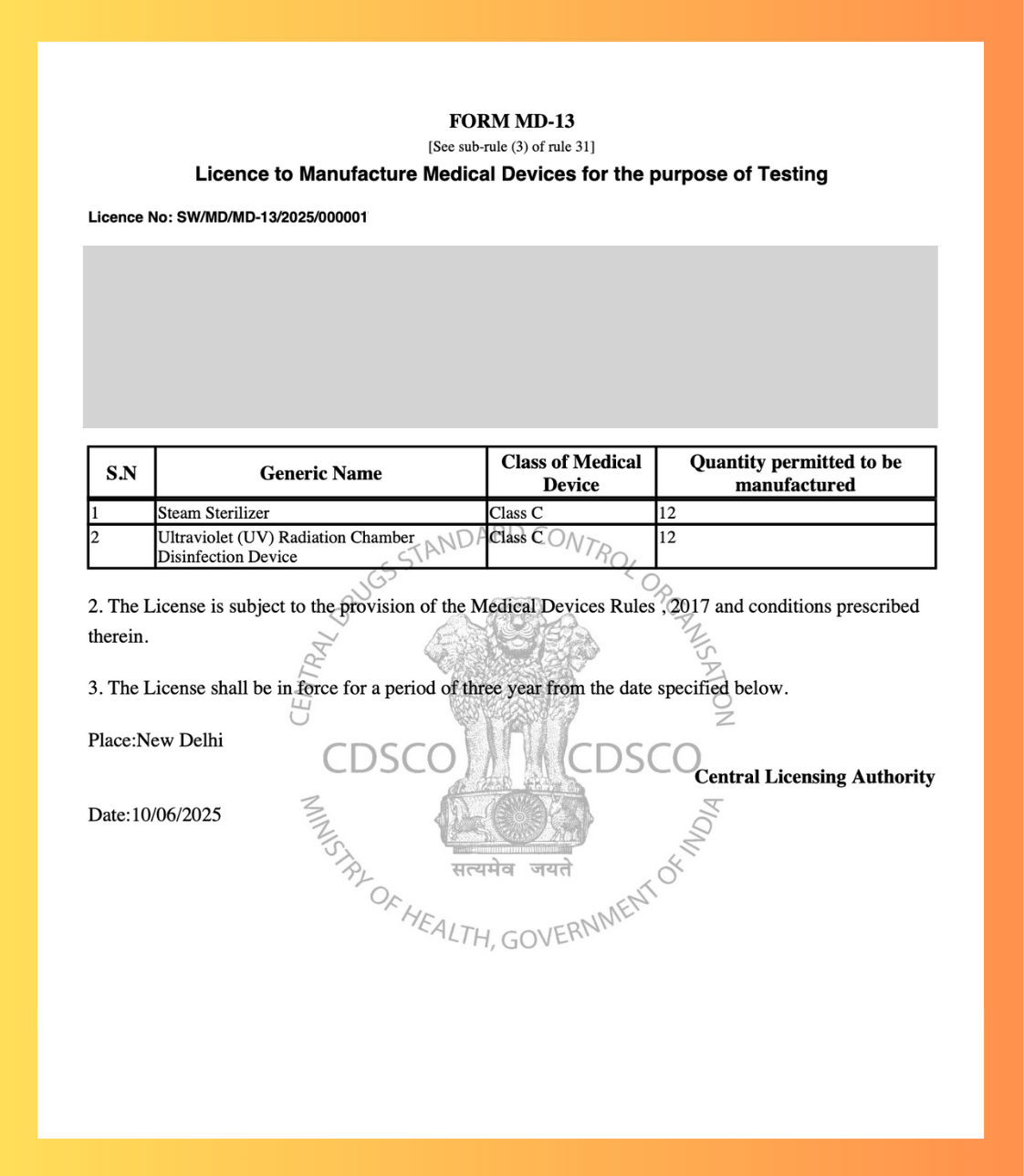
CDSCO MD-13 Test License
Get Your CDSCO MD-13 Test LicenseManufacture Medical Devices for Testing
We help you obtain the mandatory test license for manufacturing medical devices for testing or clinical investigation. This is the first step before MD5 or MD9.
Timeline
1.5-2 months (typical)
Application Form
MD-12
Authority
CDSCO HQ
Portal
NSWS
Purpose
Manufacture for Testing
Why Choose Us?
- Test license for manufacturing any class of medical device for testing/clinical investigation
- Application Form MD12 processing
- Mandatory before MD5 or MD9
- Expert guidance for compliance
Key Points
- Mandatory before MD5 or MD9
- Applied on NSWS portal
Documents Required
- Manufacturing Site address with district and Pin code
- ID Proof (Aadhar Card & Pan Card) for technical staff
- Qualification documents (Degree, All year Marks Card) for technical staff
- Experience Certificate of at least 2 years for technical staff
- List of Machines
- ISO Certificate/Approval letter/Wholesale License of any NABL Approved Certified Lab
- Product details in the Performa
- Photo of the Products
- Brief Description of Manufacturing Process of the Products
- Brief Description of Testing Process of the Products
- Flow Chart of Manufacturing Process of the Products
- Flow Chart of Testing Process of the Products
Government Fees
| Type | Fee |
|---|---|
| Per Product | ₹500 |
Frequently Asked Questions
Who needs an MD-13 Test License?
Anyone who wants to manufacture medical devices for the purpose of testing or clinical investigation in India.
How long does the MD-13 process take?
Typically 1.5-2 months.
What is the application form for MD-13?
Form MD-12, submitted via the NSWS portal.
Is MD-13 mandatory before MD5 or MD9?
Yes, you must obtain a Test License before applying for MD5 or MD9 manufacturing licenses.