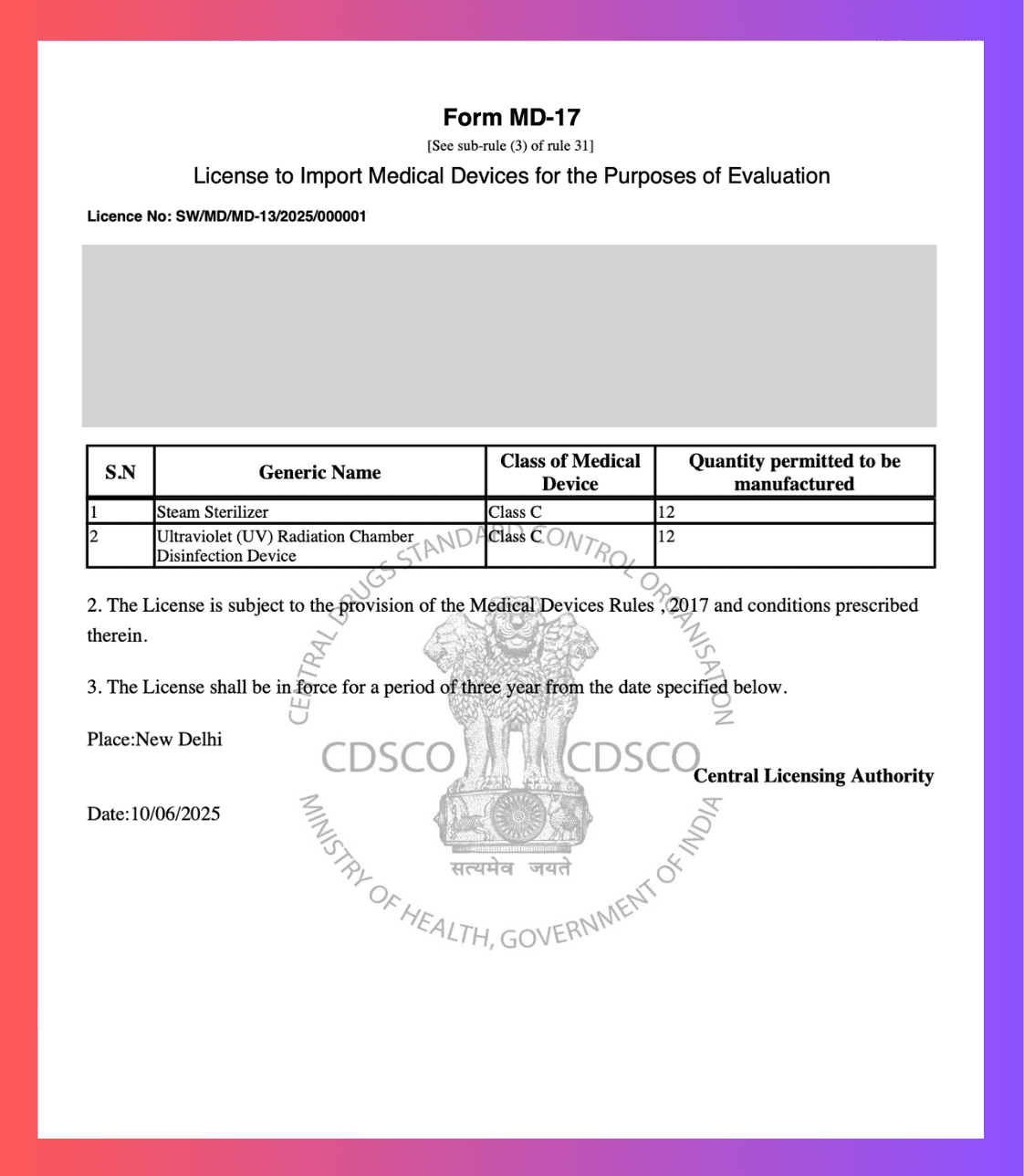
Get Your CDSCO MD-17Import Test LicenseImport Medical Devices for Testing
We help you obtain the import test license for bringing limited quantities of medical devices into India for testing, evaluation, or clinical investigation purposes.
Why Choose Our MD-17 Services?
We provide comprehensive support for your import test license application with expert guidance and proven results.
Import Limited Quantities
Import medical devices for testing or evaluation purposes
MD16 Form Processing
Expert handling of application form MD16
Clinical Trials Support
Essential for clinical trials and evaluation studies
Expert Guidance
Complete compliance and documentation support
Our 4-Step Process
We guide you through every step of the MD-17 application process with expertise and precision.
Document Preparation
We help you gather and prepare all required documents including IFU, literature, and quality certificates.
Application Submission
Submit Form MD-16 through the NSWS portal with proper justification for quantity.
CDSCO Review
CDSCO HQ reviews your application and may request additional information if needed.
License Approval
Receive your MD-17 Import Test License within approximately 2 months.
Documents Required
Ensure you have all the necessary documents ready for a smooth application process.
Government Fees
Transparent pricing with no hidden costs for your MD-17 application.
Fee Structure
Per Product
Government fee for each product imported for testing
What Our Clients Say
Don't just take our word for it - hear from professionals who have successfully obtained their MD-17 licenses.
"The MD-17 process was smooth and efficient. The team handled all documentation perfectly."
"Excellent guidance throughout the import test license application process."
Frequently Asked Questions
Get answers to the most common questions about the MD-17 Import Test License.
Who needs an MD-17 Import Test License?
How long does the MD-17 process take?
What is the application form for MD-17?
What is the fee for MD-17 license?
What documents are required for MD-17?
Can I import any quantity with MD-17?
Contact Us
Ready to Get Your MD-17 License?
Start your application today and get expert guidance throughout the entire process.