CDSCO License for Aneurysm Implant (detachable coils/clips)
Medical Device Information
Intended Use
It is intended for the endovascular embolization of intracranial aneurysms and other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae.
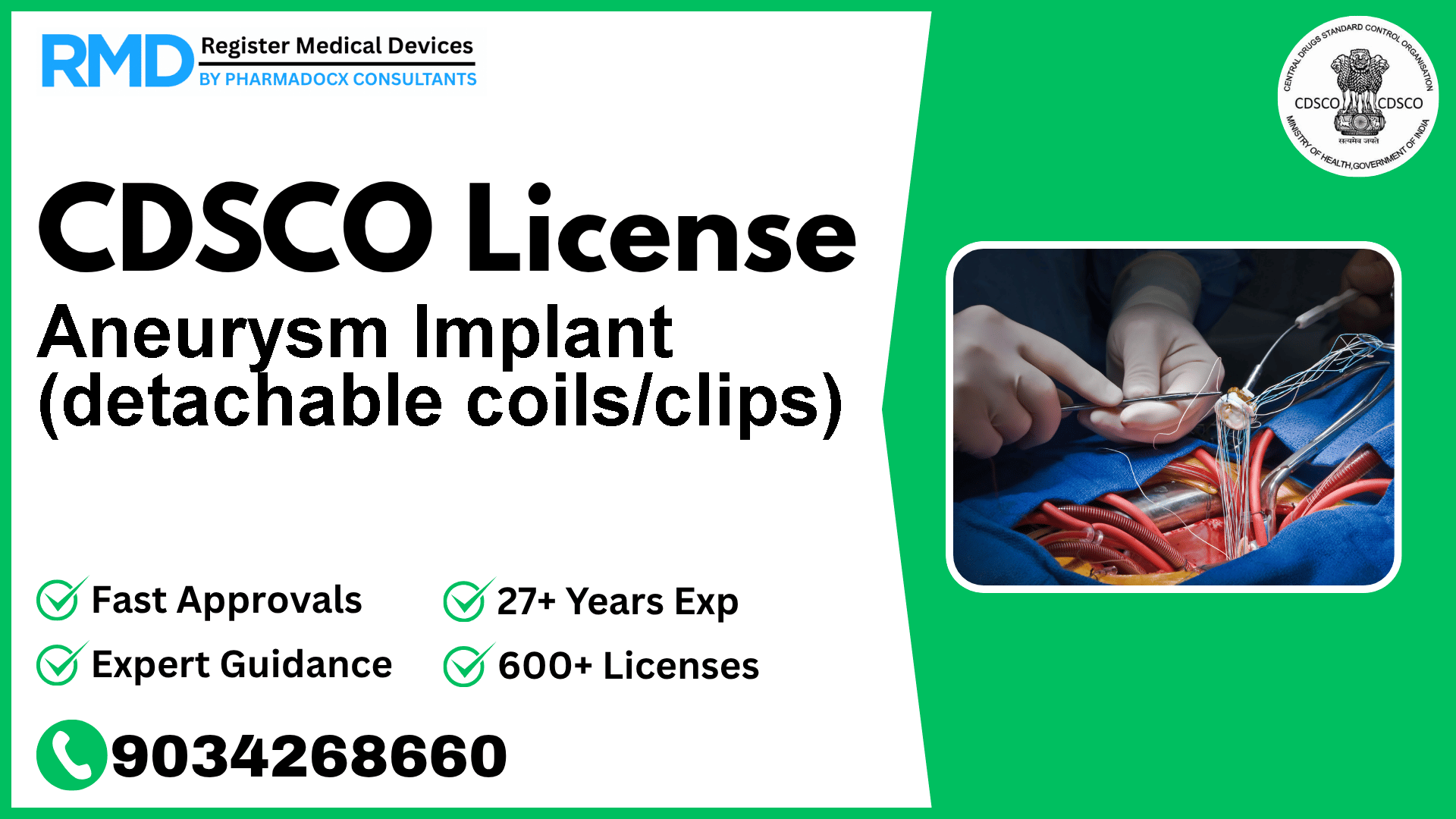
Comprehensive Guide to CDSCO Licensing for Aneurysm Implants (Class D Medical Device)
Aneurysm implants, such as detachable coils and clips, are critical medical devices designed for the endovascular embolization of intracranial aneurysms and other complex neurovascular abnormalities including arteriovenous malformations and fistulae. Classified as Class D devices under the CDSCO regulatory framework, these internal prosthetic replacements demand stringent oversight due to their high-risk nature.
With over 25 years of regulatory consulting experience and having supported more than 500 companies in obtaining CDSCO licenses, we understand the intricacies of navigating the approval process for such advanced medical devices. This guide provides you with actionable insights, detailed timelines, costs, and document requirements to streamline your CDSCO MD9 license application for aneurysm implants.
CDSCO Regulatory Framework for Aneurysm Implants
The Central Drugs Standard Control Organization (CDSCO) governs the import and manufacturing licensing for medical devices in India. Aneurysm implants fall under the Internal Prosthetic Replacements category and are notified under the regulatory notification 29/Misc/3/2017-DC (292) dated 06.06.2018.
Being a Class D device, aneurysm implants are subject to the most rigorous regulatory controls to ensure patient safety and device efficacy. The CDSCO mandates a comprehensive evaluation process including product testing, quality management system audits, and conformity assessments before issuing a manufacturing or import license.
Risk Classification and License Requirements for Class D Devices
Class D devices represent the highest risk category, involving life-supporting or life-sustaining devices where failure can result in serious health consequences or death. Aneurysm implants fall squarely in this bracket, requiring a Manufacturing License MD9 (Form MD7) granted by the Central Licensing Authority.
License Types Relevant to Aneurysm Implants:
- MD9 Manufacturing License: For domestic manufacturing of Class C and D devices.
- MD15 Import License: For importing these devices into India.
This guide focuses on the MD9 Manufacturing License process, which includes rigorous product testing and audits.
Manufacturing License Process (MD9) for Aneurysm Implants
The complete MD9 license acquisition process typically spans 4 to 5 months and involves several critical stages:
- Test License (MD13) Application: Initiate with a test license application to manufacture the device for testing purposes. This phase takes approximately 1.5 to 2 months.
- Product Testing: Conduct mandatory product testing at CDSCO-approved government laboratories. Testing ensures compliance with Indian and international standards.
- Document Preparation: Compile comprehensive technical documentation including Device Master File (DMF), Plant Master File (PMF), Risk Management File, and Quality Management System (QMS) evidence.
- Application Submission: Submit the MD9 license application (Form MD7) via the CDSCO MD Online Portal.
- Audit by CDSCO Inspectors: Undergo a detailed manufacturing site inspection.
- Query Resolution: Address any queries raised by the CDSCO or inspectors promptly.
- License Grant: Upon successful review, the MD9 license is issued.
Manufacturing License Documents Required
Preparing accurate and complete documentation is vital to avoid delays. For aneurysm implants, the following documents are mandatory:
- Company Constitution and Incorporation Certificate
- Proof of Premises Ownership or Lease Agreement
- Technical Staff Qualification and Experience Documents
- Fire NOC and Pollution Control NOC
- Device Master File (DMF): Comprehensive device-specific design and manufacturing information (Device Master File Guide)
- Plant Master File (PMF): Details of the manufacturing facility and processes (Plant Master File Guide)
- Essential Principles Checklist
- Risk Management File: Demonstrating compliance with ISO 14971 and risk mitigation (Risk Management)
- Test Reports: Results from government-approved testing laboratories (Testing Laboratories)
- Labels and Instructions for Use (IFU)
- Quality Management System Documents: ISO 13485 certification and SOPs
Ensuring these documents are precise and aligned with CDSCO expectations significantly reduces audit queries and expedites approval.
Import License Process (MD15) for Aneurysm Implants
For manufacturers or distributors aiming to import aneurysm implants, the MD15 Import License application is submitted to the Central Licensing Authority.
This process generally takes 5 to 6 months and does not require a test license. Key steps include:
- Preparing import-specific documentation including Manufacturing License from the country of origin, Free Sale Certificate, CE Certificate (if applicable), and ISO 13485:2016 certification.
- Submitting the application on the CDSCO MD Online Portal.
- Responding to departmental queries.
- Receipt of the MD15 license.
Documents required include:
- Manufacturing License for the device from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate
- Device Master File
- Plant Master File
- Wholesale License
- Company Constitution
For detailed guidance, refer to our Import License Guide.
Timeline and Processing Duration
| Process Step | Approximate Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | 2 – 3 weeks |
| Application Submission | Immediate after prep |
| CDSCO Inspection & Audit | 3 – 4 weeks |
| Query Resolution | 2 – 4 weeks |
| License Grant (MD9) | Total 4 – 5 months |
Being proactive in document preparation and prompt in responding to queries can significantly reduce total processing time.
Government Fees and Costs
The government fees for the MD9 license are as follows:
- Application Fee: ₹50,000 per application
- Per Product Fee: ₹1,000 per product
Additional costs include:
- Testing laboratory charges
- Fees for third-party notified body audits
The list of Notified Bodies authorized for conducting CDSCO audits is available online.
Common Challenges and Practical Solutions
Challenge 1: Delays in Product Testing
- Solution: Engage early with CDSCO-approved testing labs to schedule tests and clarify requirements upfront.
Challenge 2: Incomplete or Non-Compliant Documentation
- Solution: Use standardized templates and cross-check documents against CDSCO checklists and regulatory updates.
Challenge 3: Audit Non-conformities
- Solution: Conduct internal mock audits and ensure your QMS aligns with ISO 13485 and CDSCO guidelines.
Challenge 4: Query Resolution Delays
- Solution: Assign a dedicated regulatory liaison to prepare timely and detailed responses.
Expert Consultation and Support
Navigating the CDSCO regulatory landscape for Class D devices requires technical expertise and regulatory know-how. Our team has successfully facilitated over 500 CDSCO license approvals, including complex aneurysm implants.
We offer end-to-end support encompassing:
- Regulatory strategy and classification
- Document preparation and review
- Coordination with notified bodies and testing labs
- Application submission and follow-up
- Training on CDSCO compliance and audit readiness
Getting Started with Your CDSCO License Application
- Assess Device Classification: Confirm your device is Class D under CDSCO’s Medical Device Classification.
- Prepare Technical Documentation: Collect and prepare your DMF, PMF, risk management files, and QMS documents.
- Apply for Test License (MD13): Submit your test license application via the CDSCO MD Online Portal.
- Schedule Product Testing: Coordinate with CDSCO-approved labs early to avoid bottlenecks.
- Submit MD9 License Application: After successful testing, file your manufacturing license application (Form MD7) online.
- Prepare for Audit: Conduct internal audits and ensure facility readiness.
- Respond Promptly to Queries: Maintain clear communication with CDSCO throughout the process.
Embarking on your CDSCO licensing journey with a clear roadmap and expert support will position your aneurysm implant product for timely market entry in India. Reach out to us to leverage our extensive experience and ensure a smooth regulatory approval process.