CDSCO License for Angiographic Catheter
Medical Device Information
Intended Use
Designed to provide a pathway for delivering contrast media to selected sites in the device vascular system including the carotid arteries.
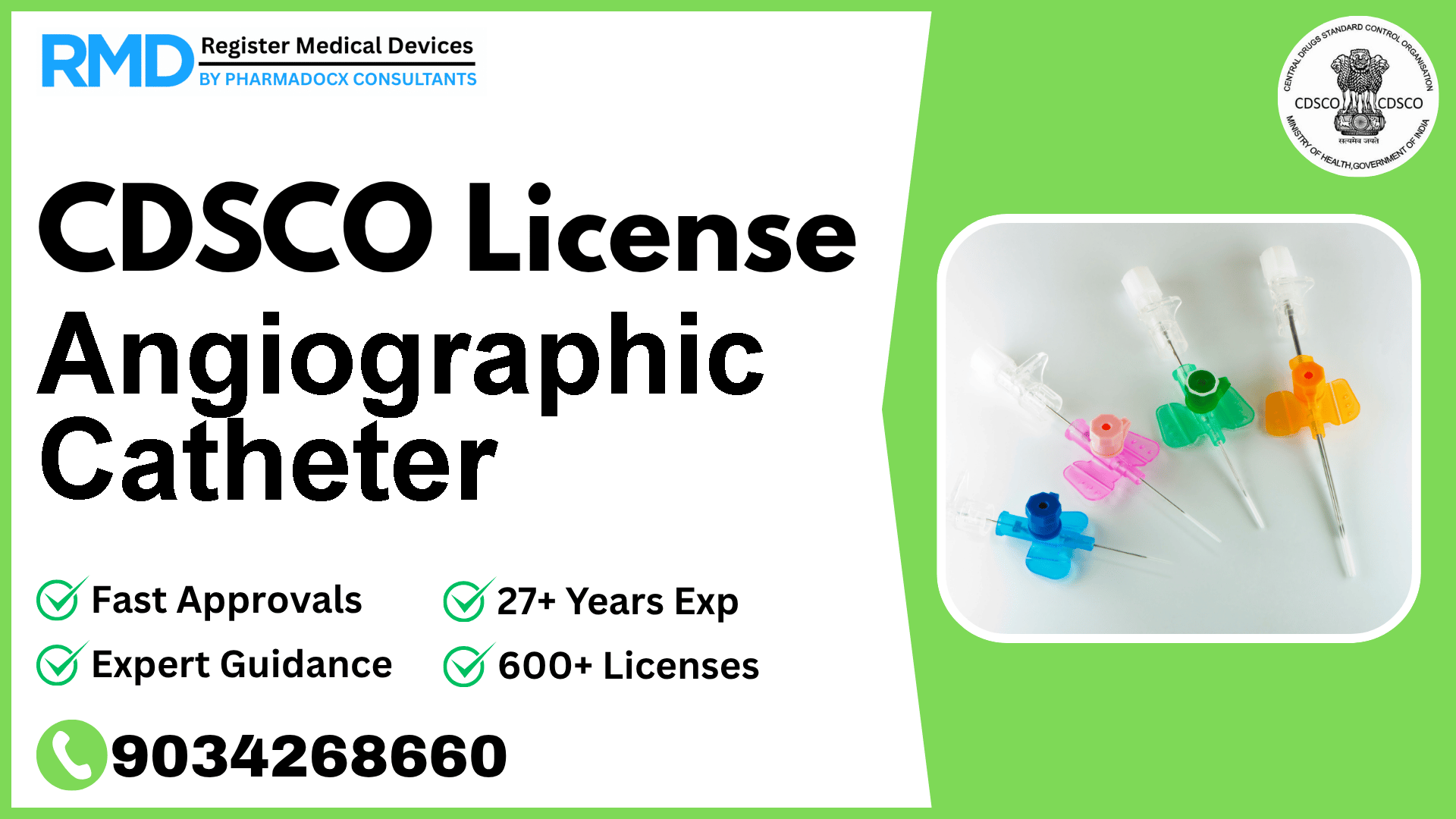
Comprehensive Guide to CDSCO Licensing for Angiographic Catheters (Class B Medical Device)
Angiographic catheters are specialized medical devices designed to provide a safe and effective pathway for delivering contrast media to selected sites within the vascular system, including critical areas such as the carotid arteries. Given their direct interaction with the human vascular system, these devices fall under Risk Class B as per the CDSCO classification. Ensuring compliance with CDSCO regulations is paramount for manufacturers and importers aiming to market angiographic catheters in India.
With over 25 years of expertise and having successfully assisted more than 500 companies, we understand the nuances and challenges in navigating the CDSCO licensing process for medical devices like angiographic catheters. This detailed guide covers everything from regulatory framework to practical next steps for obtaining your manufacturing or import license.
CDSCO Regulatory Framework for Angiographic Catheters
Angiographic catheters are regulated under the Medical Device Rules (MDR) 2017 notified by the CDSCO (Central Drugs Standard Control Organization). As per the notification [29/Misc/3/2017-DC (292)] dated 06.06.2018, these devices are classified as Class B, requiring a manufacturing license known as MD5, granted by the State Licensing Authority.
The regulatory framework mandates strict adherence to quality standards, product testing, risk management, and detailed documentation to ensure patient safety and device efficacy.
Risk Classification and License Requirements
- Risk Class: B (Low to Moderate Risk)
- Applicable License: MD5 Manufacturing License
- License Authority: State Licensing Authority
- Application Form: MD3 for MD5 License
- Test License Requirement: Mandatory MD13 Test License prior to final license application
Manufacturers must first obtain a Test License (Form MD13), conduct product testing at CDSCO-approved laboratories, submit a comprehensive application including audit reports, and finally receive the manufacturing license (Form MD5).
Manufacturing License Process (MD5) for Angiographic Catheters
The MD5 license process is a multi-step procedure:
- Apply for Test License (Form MD13): This initial step permits manufacturing for testing purposes.
- Product Testing: Conduct tests at government-approved labs to validate safety and performance.
- Document Preparation: Compile all necessary technical and regulatory documents.
- Submit Manufacturing License Application (Form MD3): Application for MD5 license via the CDSCO MD Online Portal.
- Audit by Notified Body: A mandatory audit by a notified body listed here.
- Address Queries: Respond to any clarifications or observations raised during the audit or by CDSCO officials.
- License Grant: Upon satisfactory review, the MD5 license is granted.
Manufacturing License Documents Required for Angiographic Catheters
To ensure a smooth application, prepare the following key documents:
- Company Constitution (e.g., Memorandum & Articles of Association)
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Details of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design, manufacturing processes, and specifications (see our detailed guide)
- Plant Master File (PMF) describing the manufacturing facility and quality management system (learn more)
- Essential Principles Checklist confirming compliance with MDR 2017
- Risk Management File illustrating risk analysis and mitigation (risk management insights)
- Test Reports from CDSCO-approved laboratories (find labs here)
- Labels, Instructions for Use (IFU), and packaging details
- Quality Management System (QMS) documentation, typically ISO 13485:2016 compliant
Import License Process (MD15) for Angiographic Catheters
If you are an importer seeking to bring angiographic catheters into India, an Import License (MD15) issued by the Central Licensing Authority is mandatory. The process includes:
- Document preparation including Manufacturing License from the country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE Certificate, and related master files.
- Application submission on the CDSCO MD Online Portal.
- Query resolution and final issuance of the MD15 license.
Note that unlike MD5, the import license process does not require a test license but demands comprehensive documentation proving compliance and product safety.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certification
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License (if applicable)
- Company Constitution Documents
Timeline and Processing Duration
MD5 Manufacturing License (Class B Angiographic Catheter): Approximately 3 to 4 months
- Test License (MD13): 1.5 to 2 months
- Product Testing: 2 to 3 weeks depending on lab
- Application Review & Audit: 1 to 1.5 months
MD15 Import License: Approximately 5 to 6 months
Government Fees and Costs
MD5 License:
- Application Fee: ₹5,000
- Per Product Fee: ₹500
MD15 Import License for Class B:
- Site Fee: $2,000
- Per Product Fee: $1,000
Additional costs involve product testing fees at approved laboratories and audit fees charged by notified bodies.
Common Challenges and Solutions
Challenge: Delays in product testing due to limited slots at government labs.
Solution: Plan testing well in advance and leverage multiple approved labs to reduce wait time.
Challenge: Incomplete or non-compliant documentation causing query back-and-forth.
Solution: Utilize expert consultation to prepare comprehensive Device and Plant Master Files based on CDSCO norms.
Challenge: Audit non-conformities relating to QMS or facility standards.
Solution: Conduct internal pre-audit assessments and align with notified body requirements ahead of the official audit.
Expert Consultation and Support
Having guided over 500 manufacturers and importers, we provide tailored support including:
- Pre-application gap analysis
- Documentation drafting and review
- Coordination with notified bodies and testing labs
- Handling CDSCO queries and audit facilitation
Our in-depth experience ensures you avoid common pitfalls and expedite your license approval.
Getting Started with Your CDSCO License Application
- Determine Your Device Classification: Confirm Class B status using the Medical Device Classification guide.
- Register on CDSCO MD Online Portal: Create your account at https://cdscomdonline.gov.in/.
- Apply for Test License (MD13): Initiate the process to manufacture samples for testing.
- Engage a CDSCO-Approved Testing Laboratory: Schedule testing and gather reports.
- Prepare Full Documentation Set: Utilize expert help to compile and review your Device Master File, Plant Master File, and supporting documents.
- Apply for MD5 License (Form MD3): Submit your application online.
- Coordinate Audit and Respond to Queries: Prepare for notified body audit and timely response to CDSCO.
By following these actionable steps with expert guidance, you can confidently navigate the CDSCO licensing process for your angiographic catheters and successfully enter the Indian market.
For detailed assistance, explore our comprehensive MD5 License Guide and get in touch with our regulatory consultants today.