CDSCO License for Angiographic Guide Wire
Medical Device Information
Intended Use
It delivers radio opaque media and therapeutic agents to selected sites in the vascular system. It is also used to lead a guide wire or a catheter into the target site.
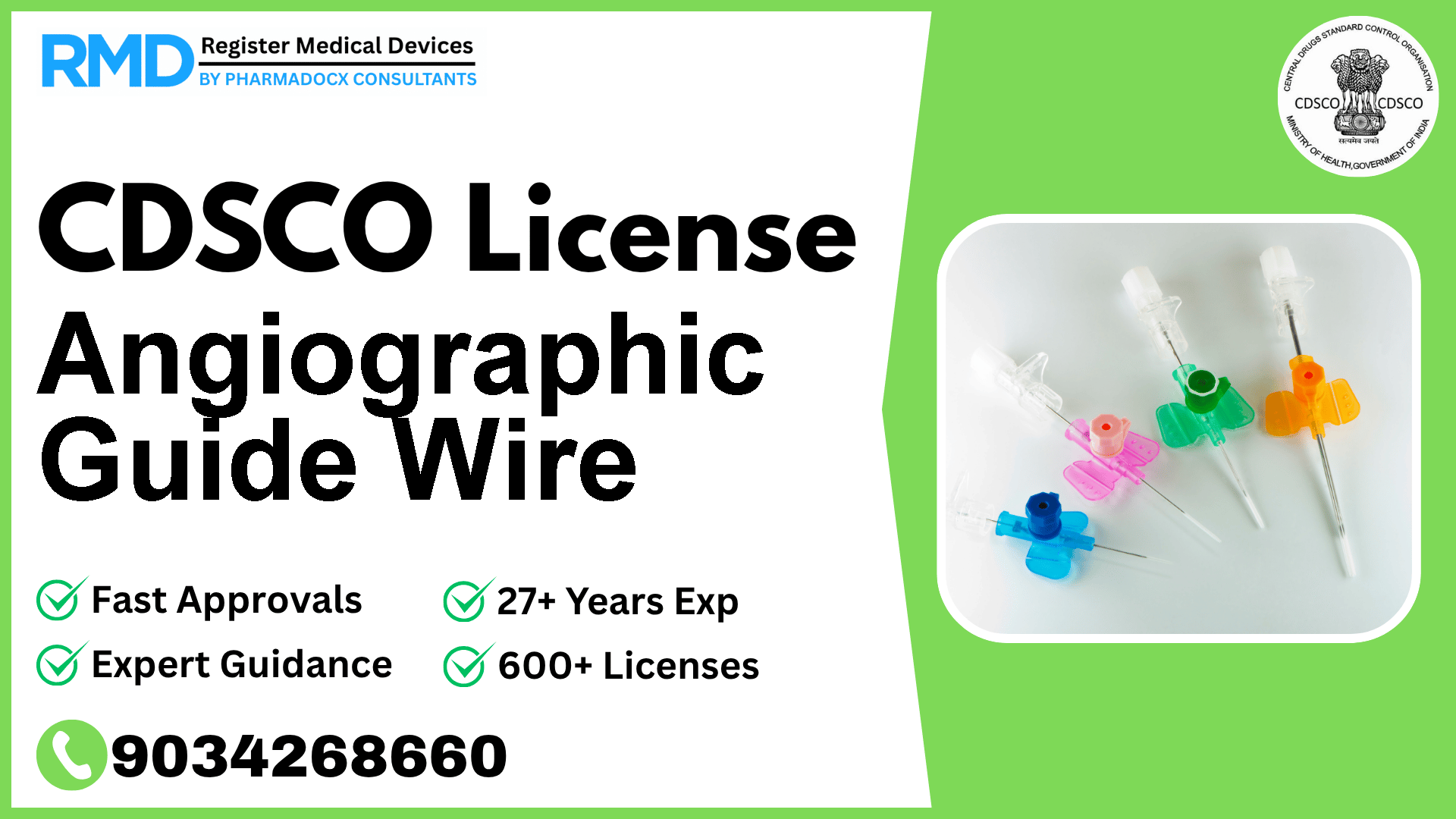
Comprehensive CDSCO License Guide for Angiographic Guide Wire (Class D Medical Device)
As a trusted regulatory consultancy with over 25 years of experience and more than 500 successful CDSCO licensing cases, we understand the complexities manufacturers and importers face when seeking market authorization for high-risk devices like the Angiographic Guide Wire. This vascular catheter device, classified as Class D under CDSCO guidelines, plays a critical role in delivering radiopaque media and therapeutic agents to targeted vascular sites, making regulatory compliance indispensable.
CDSCO Regulatory Framework for Angiographic Guide Wire
The Central Drugs Standard Control Organization (CDSCO) governs the regulatory framework for medical devices in India, ensuring safety, efficacy, and quality. The Angiographic Guide Wire is categorized as a Class D device due to its invasive nature and potential risk, requiring the most stringent scrutiny. Compliance with the notification 29/Misc/3/2017-DC (292) dated 06.06.2018 is mandatory.
Risk Classification and License Requirements
Class D devices, including the Angiographic Guide Wire, are high-risk and thus require a Manufacturing License (MD9) or Import License (MD15) issued by the Central Licensing Authority. The MD9 license governs manufacturing within India, while MD15 covers imports. Both licenses demand rigorous evaluation, testing, and documentation.
Manufacturing License Process for Class D Devices (MD9)
Manufacturers must secure an MD9 license via the following steps:
- Test License Application (Form MD13): Obtain a test license valid for 12 months. This permits sample testing but does not authorize commercial manufacturing. Processing time is approximately 1.5 to 2 months.
- Product Testing: Samples must be tested at CDSCO-approved government laboratories listed on the Testing Laboratories portal. Testing covers biocompatibility, sterility, mechanical performance, and other essential parameters.
- Document Preparation: Compile required documents including technical files, Device Master File (DMF), and Plant Master File (PMF).
- Application Submission (Form MD7): Apply for the MD9 license through the CDSCO MD Online Portal.
- Audit & Inspection: CDSCO inspectors conduct a detailed audit of manufacturing premises and quality management systems.
- Query Resolution: Address any observations or requests for additional information by the authorities.
- Grant of License (Form MD9): Upon successful evaluation, the license is granted.
The entire process typically takes 4 to 5 months, depending on document readiness and audit scheduling.
Manufacturing License Documents Required for Angiographic Guide Wire
For a Class D catheter device, the following documents are essential:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualification Certificates of Technical Staff
- Fire and Pollution No Objection Certificates (NOCs)
- Device Master File (DMF) detailing design, specifications, and manufacturing processes (see our DMF guide)
- Plant Master File (PMF) documenting manufacturing facilities and quality controls (see PMF guide)
- Essential Principles Checklist demonstrating conformity to regulatory safety and performance standards
- Risk Management File compliant with ISO 14971 principles (risk management insights)
- Test Reports from CDSCO-approved laboratories
- Product Labeling, Instructions for Use (IFU), and Packaging Details
- Quality Management System (QMS) documentation, typically ISO 13485:2016 certification
Import License Process for Class D Devices (MD15)
Importers must apply for the MD15 import license through these steps:
- Document Compilation: Gather all required documents including a valid manufacturing license from the country of origin, Free Sale Certificate, ISO 13485 certificate, CE certificate if applicable, DMF, PMF, and Wholesale License.
- Application Submission (Form MD14): Submit your application online via the CDSCO MD Online Portal.
- Review and Queries: The CDSCO evaluates documents and may request additional clarifications.
- Grant of Import License (Form MD15): Upon successful review, the license is issued.
This process generally takes 5 to 6 months.
Import License Documents Required for Angiographic Guide Wire
- Valid Manufacturing License from the country of origin
- Free Sale Certificate ensuring product marketing approval in the manufacturing country
- ISO 13485:2016 Quality Management System Certificate
- CE Certificate (if marketed in Europe)
- Device Master File (DMF)
- Plant Master File (PMF)
- Wholesale License for distribution in India
- Company Constitution and Incorporation Certificates
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 4 - 6 weeks |
| Document Preparation | Variable (1-2 months typical) |
| License Application Processing (MD9 / MD15) | 3 - 4 months (MD9), 5 - 6 months (MD15) |
| Total Estimated Time | 4 - 5 months (Manufacturing), 5 - 6 months (Import) |
Government Fees and Costs
- MD9 Manufacturing License:
- Application Fee: Rs 50,000
- Per Product Fee: Rs 1,000 (applicable for each variant)
- MD15 Import License:
- Class D Devices: $3,000 per site
- $1,500 per product
Additional costs include testing fees at government-approved labs and expenses related to audits by notified bodies or CDSCO inspectors.
Common Challenges and Solutions
- Delayed Testing Results: Testing at government labs can face backlogs. To mitigate delays, submit samples promptly and consider parallel documentation preparation.
- Incomplete Documentation: Missing or inconsistent data often leads to query letters. Use detailed checklists like our Device Master File guide to ensure completeness.
- Audit Non-Compliance: Premises or QMS deficiencies can hinder license approval. Engage experienced consultants for pre-audit assessments.
- Regulatory Updates: Stay current with CDSCO notifications. The official CDSCO MD Online Portal is the best resource for updates.
Expert Consultation and Support
Navigating the CDSCO licensing maze for Class D devices demands expertise. Our seasoned consultants provide:
- Tailored gap analyses for your manufacturing/importing setup
- Assistance with DMF and PMF preparation
- Coordination with notified bodies listed on the Notified Bodies portal for audits
- Guidance through testing and audit phases
- Complete application filing and follow-up
Getting Started with Your CDSCO License Application
To initiate your licensing journey for the Angiographic Guide Wire:
- Evaluate Device Classification: Confirm Class D status using CDSCO guidelines or our Medical Device Classification resource.
- Prepare Technical Documentation: Begin compiling your DMF, PMF, risk management files, and QMS certificates.
- Apply for Test License (MD13): Submit your application for test license via the CDSCO MD Online Portal.
- Coordinate Product Testing: Arrange sample testing at approved labs to avoid delays.
- Plan for Audit: Schedule pre-audit consultations and prepare your facility.
- File for Manufacturing License (MD9): Post successful testing, submit the manufacturing license application.
For importers, focus on gathering foreign manufacturing approvals, certificates, and apply for the MD15 license accordingly.
Embarking on this path with expert guidance ensures a smoother regulatory process, faster market access, and compliance confidence. Contact us today to leverage our 25+ years of regulatory expertise for your Angiographic Guide Wire licensing needs.