CDSCO License for Balloon Repair Kit Catheter
Medical Device Information
Intended Use
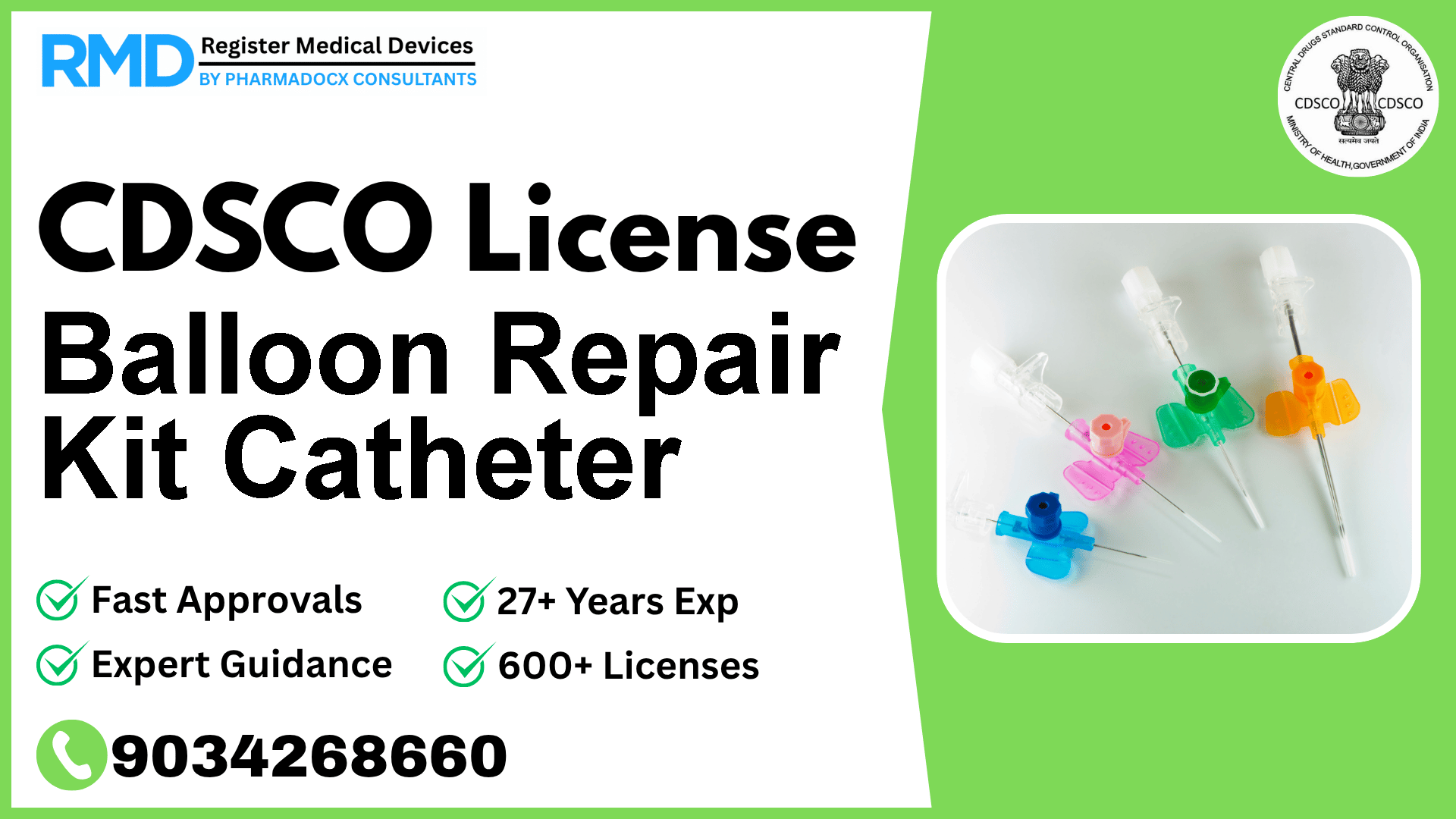
A device used to repair or replace the balloon of a balloon catheter. The kit contains the materials, such as glue and balloons, necessary to affect the repair or replacement.
Introduction to Balloon Repair Kit Catheter and Regulatory Importance
The Balloon Repair Kit Catheter is a specialized medical device intended for repairing or replacing the balloon component of balloon catheters. This kit typically contains essential materials like glue and replacement balloons, ensuring the catheter remains functional without the need for complete replacement. Given its critical role in patient care, regulatory compliance with India's Central Drugs Standard Control Organization (CDSCO) is mandatory to ensure safety, quality, and efficacy.
As specialists with over 25 years of regulatory consulting experience, having assisted 500+ medical device companies, we understand the complexities involved in obtaining a CDSCO license for risk Class C devices like the Balloon Repair Kit Catheter. Navigating the regulatory framework efficiently can save months and reduce costs.
CDSCO Regulatory Framework for Balloon Repair Kit Catheter
The CDSCO regulates medical devices under the Medical Device Rules (MDR) 2017. The Balloon Repair Kit Catheter falls under the catheter category, notified under Notification 29/Misc/3/2017-DC (292) dated 06.06.2018. As a Class C device, it is subject to rigorous regulatory scrutiny due to its medium to high risk profile.
The manufacturing and import of Class C devices require a Central Licensing Authority approval, specifically through the MD9 manufacturing license for local production or MD15 import license for imported devices.
Risk Classification and License Requirements
The Balloon Repair Kit Catheter is designated as a Class C device. According to the CDSCO risk classification:
- Class A/B devices follow a simpler state-level MD5 license process.
- Class C/D devices require the more stringent central-level MD9 manufacturing license.
This classification mandates compliance with higher standards, including comprehensive testing, plant audits, and detailed documentation.
Manufacturing License Process for Balloon Repair Kit Catheter (MD9 License)
For manufacturers intending to produce the Balloon Repair Kit Catheter in India, the MD9 license process is obligatory. The process involves:
- Test License Application (Form MD13): Obtain a test license allowing product testing; this stage typically takes 1.5 to 2 months.
- Product Testing: Conduct testing at CDSCO-approved laboratories. Testing ensures compliance with Indian and international standards.
- Document Preparation: Compile necessary technical and compliance documentation.
- MD9 License Application (Form MD7): Submit the manufacturing license application via the CDSCO MD Online Portal.
- Audit by CDSCO Inspectors: Central licensing authority conducts a thorough audit of manufacturing premises and quality systems.
- Query Resolution: Address any queries raised during audit or document review.
- License Grant (Form MD9): Upon satisfactory compliance, the license is granted.
The entire process generally takes approximately 4 to 5 months.
Manufacturing License Documents Required for MD9
Preparing a complete and accurate dossier is crucial for timely approval. For the Balloon Repair Kit Catheter, you must submit:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualifications and Experience Details
- Fire and Pollution No Objection Certificates (NOCs)
- Detailed Device Master File (DMF) covering design, specifications, and manufacturing processes. Our comprehensive Device Master File guide can assist you.
- Plant Master File (PMF) describing manufacturing infrastructure, quality systems, and environmental controls. Learn how to create an effective PMF here.
- Essential Principles Checklist ensuring compliance with safety and performance standards
- Risk Management File demonstrating hazard identification and mitigation strategies (refer to our Risk Management guide)
- Test Reports from CDSCO-approved laboratories, certifying device compliance
- Labels and Instructions for Use (IFU) in English and regional languages
- Quality Management System (QMS) documents, preferably ISO 13485:2016 certification
Import License Process for Balloon Repair Kit Catheter (MD15 License)
For importers, the MD15 license is required to legally bring the Balloon Repair Kit Catheter into India. The process includes:
- Document Preparation: Gather all required certificates and licenses.
- License Application (Form MD14): Submit online through the CDSCO MD Online Portal.
- Query Resolution: Respond promptly to any departmental queries.
- License Issuance (Form MD15): Upon clearance, the import license is granted.
The import license process typically takes 5 to 6 months.
Import License Documents Required for MD15
The import license demands a comprehensive set of documents:
- Valid Manufacturing License from the country of origin
- Free Sale Certificate indicating product approval in the exporting country
- ISO 13485:2016 certification
- CE Certificate or equivalent regulatory approval
- Device Master File and Plant Master File
- Wholesale Drug License (if applicable)
- Company Constitution and Registration Documents
Timeline and Processing Duration for Balloon Repair Kit Catheter Licensing
| License Type | Approximate Duration | Key Steps Included |
|---|---|---|
| MD9 Manufacturing | 4-5 months | Test license, testing, audit, queries |
| MD15 Import | 5-6 months | Document review, application, queries |
Planning your submission with these timelines in mind ensures smoother regulatory compliance and market entry.
Government Fees and Costs Breakdown
- MD9 Manufacturing License:
- Application Fee: Rs 50,000
- Per Product Fee: Rs 1,000
- MD15 Import License:
- Class C Device Fees: Rs 3,000 per site
- Rs 1,500 per product
Additional costs may include testing laboratory fees and consultancy charges if you engage expert support.
Common Challenges and Practical Solutions
- Delays in Document Preparation: We recommend early engagement with expert consultants to prepare Device and Plant Master Files accurately.
- Testing Bottlenecks: Utilize the updated list of CDSCO-approved testing laboratories to schedule timely product testing.
- Audit Non-Compliance: Conduct pre-audit internal reviews and gap assessments. Familiarize yourself with the list of notified bodies for guidance.
- Query Resolution Delays: Maintain transparent and timely communication with CDSCO to avoid prolonged back-and-forth.
Expert Consultation and Support
With extensive experience in navigating CDSCO licensing for over 500 clients, we provide tailored end-to-end regulatory consulting services. From documentation to audit readiness and application submission, our expertise ensures you avoid common pitfalls and accelerate market entry.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm the Balloon Repair Kit Catheter is Class C using our Medical Device Classification resource.
- Prepare Your Documentation: Start assembling the Device Master File, Plant Master File, and Risk Management File.
- Apply for Test License (For Manufacturing): Submit Form MD13 via the CDSCO MD Online Portal.
- Schedule Product Testing: Engage CDSCO-approved labs early to avoid delays.
- Plan for Audit: Organize your manufacturing site and QMS for inspection.
- Submit Final Application: File Form MD7 for MD9 license or Form MD14 for MD15 license through the online portal.
Our team is ready to assist you at every step to ensure your Balloon Repair Kit Catheter gains timely regulatory approval, enabling you to serve the Indian healthcare market confidently and compliantly.