CDSCO License for Chorionic Villus Sampling Catheter
Medical Device Information
Intended Use
An ultrasound guides a thin catheter through the cervix to your placenta. The chorionic villi cells are gently suctioned into the catheter.
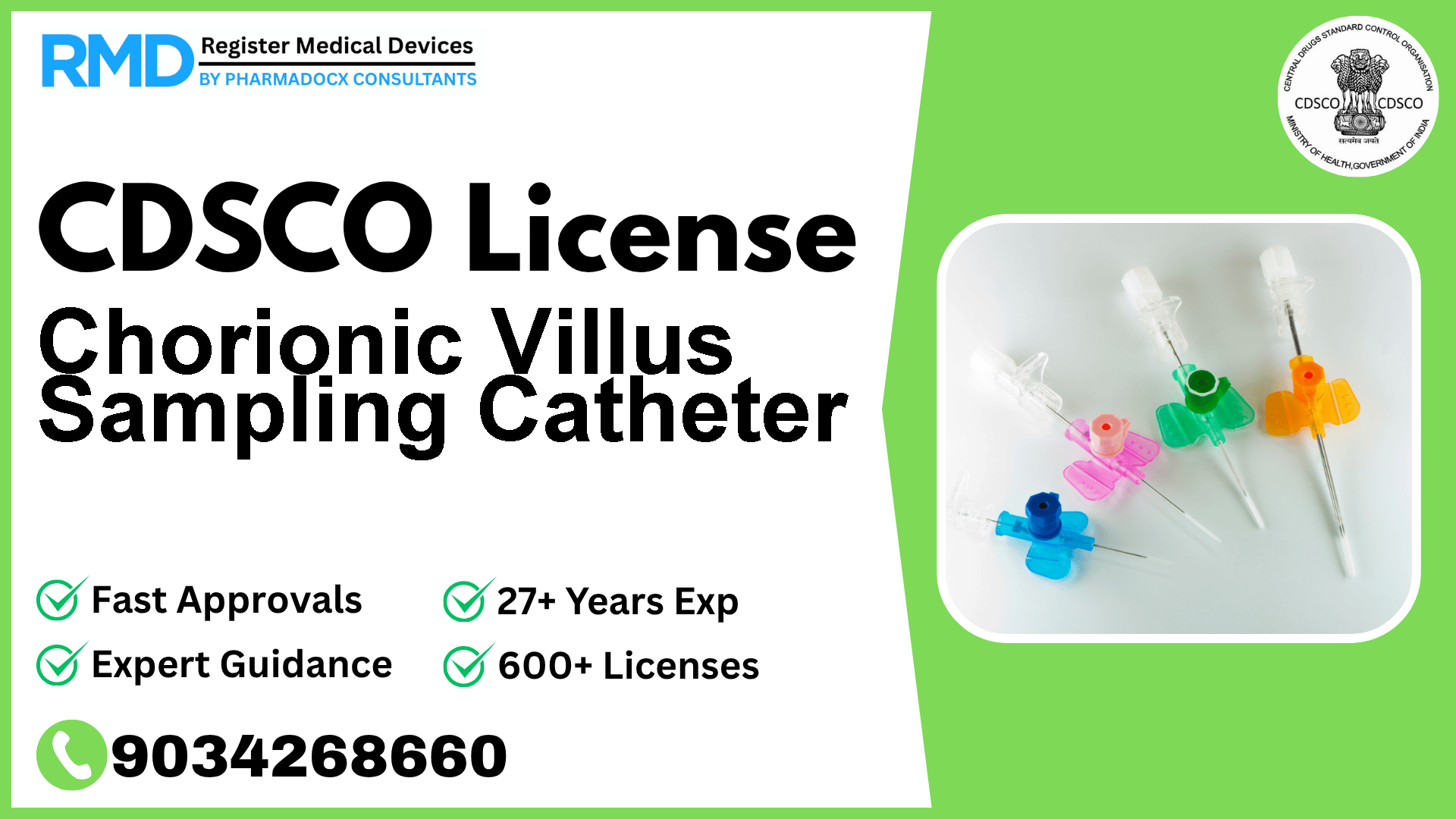
Comprehensive Guide to CDSCO Licensing for Chorionic Villus Sampling Catheters (Class B Medical Device)
As seasoned regulatory consultants with over 25 years of experience and having successfully guided 500+ manufacturers and importers through the Indian medical device regulatory landscape, we understand the unique challenges and requirements involved in obtaining CDSCO licenses for devices such as the Chorionic Villus Sampling (CVS) Catheter. This specialized catheter, classified as a Class B medical device under CDSCO regulations, plays a critical role in prenatal diagnostics by enabling the gentle suction of chorionic villi cells via ultrasound guidance.
CDSCO Regulatory Framework for Chorionic Villus Sampling Catheters
The Central Drugs Standard Control Organization (CDSCO) governs the regulation of medical devices in India, ensuring safety, efficacy, and quality. The CVS Catheter falls under the catheter category notified under Notification 29/Misc/3/2017-DC (292) dated 06.06.2018, making compliance with CDSCO’s licensing and quality requirements mandatory before market entry.
Risk Classification and License Requirements for Class B Devices
Chorionic Villus Sampling Catheters are classified as Class B devices, indicating a low to moderate risk profile. As per CDSCO guidelines, manufacturing these requires an MD5 license, issued by the State Licensing Authority. This classification impacts the documentation, audit process, and fee structure.
For a detailed understanding of medical device classification, refer to our Medical Device Classification guide.
Manufacturing License Process (MD5) for CVS Catheters
The MD5 license application process involves several sequential steps:
Test License Application (Form MD13): Initiate by applying for a test license to manufacture the CVS catheter prototype for testing purposes. This step typically takes 1.5 to 2 months.
Product Testing: The catheter must be tested at government-approved laboratories for compliance with essential performance and safety standards. A list of such labs is available on the CDSCO Testing Laboratories page.
Document Preparation: Compile comprehensive documentation, including Device Master File, Plant Master File, risk management, and quality management system (QMS) documents.
License Application Submission (Form MD3): File your MD5 license application through the CDSCO MD Online Portal.
Audit by Notified Body: An audit is conducted by a notified body listed here. This audit assesses your manufacturing premises, QMS, and compliance with regulatory requirements.
Queries and Clarifications: Address any queries raised by the licensing authority or notified body promptly.
Grant of License (Form MD5): Upon successful audit and document verification, the MD5 manufacturing license is granted.
For an in-depth walkthrough, consult our MD5 License Guide.
Manufacturing License Documents Required for CVS Catheters
Accurate and complete documentation is critical for smooth approval. For Class B devices like the CVS catheter, you must submit:
- Company Constitution (Incorporation Certificate, Memorandum & Articles of Association)
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire and Pollution No Objection Certificates (NOCs)
- Device Master File (DMF) detailing design, manufacturing, and performance specifications (Device Master File Guide)
- Plant Master File (PMF) outlining manufacturing infrastructure and processes (Plant Master File Guide)
- Essential Principles Checklist demonstrating compliance with safety and performance requirements
- Risk Management File per ISO 14971 standards (Risk Management Insights)
- Test Reports from CDSCO-recognized laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documents (ISO 13485:2016 compliance)
Import License Process (MD15) for CVS Catheters
If you are an importer of CVS catheters, obtaining an MD15 license from the Central Licensing Authority is mandatory. This process generally takes 5-6 months and does not require a test license.
Steps include:
Preparation of comprehensive documentation including Manufacturing License from the country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE Certificate (if applicable), Device Master and Plant Master Files, and Wholesale License.
Submission of application on CDSCO MD Online Portal.
Resolution of any queries raised by the licensing authority.
Grant of Import License (Form MD15).
For detailed guidance, see our Import License Guide.
Import License Documents Required
- Manufacturing License from the exporting country
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale License in India
- Company Constitution
Timeline and Processing Duration
| Process Step | Approximate Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | Concurrent with testing |
| License Application & Audit | 1 – 1.5 months |
| Query Resolution & Grant | 0.5 – 1 month |
Total Duration: Approximately 3-4 months for manufacturing license (MD5) for Class B devices like CVS catheters.
Government Fees and Costs
- MD5 License: Rs 5,000 per application + Rs 500 per product.
- Test License (MD13): Included in overall process, fees vary by state.
These fees exclude costs associated with product testing, audit fees charged by notified bodies, and document preparation consultancy.
Common Challenges and Practical Solutions
Incomplete Documentation: Ensure all files such as Device Master File and Risk Management File are meticulously prepared. Utilize professional templates and expert consultation.
Delays in Product Testing: Engage early with government-approved labs (Testing Laboratories) to schedule testing in advance.
Non-compliance Findings During Audit: Pre-audit internal assessments can identify gaps, allowing corrective actions before notified body visits.
Query Resolution Delays: Maintain proactive communication with CDSCO officials and respond promptly to queries.
Expert Consultation and Support
Our team has extensive experience navigating the CDSCO regulatory system specifically for devices like the Chorionic Villus Sampling Catheter. We provide end-to-end support including:
- Gap analysis of your current documentation
- Preparation of Device and Plant Master Files
- Coordination with notified bodies and testing labs
- Liaising with CDSCO officials for streamlined query handling
- Timely application submissions via the CDSCO MD Online Portal
Getting Started with Your CDSCO License Application
Assess Your Device Classification: Confirm your device as Class B and eligible for MD5 license.
Prepare Test License Application (MD13): Submit through the CDSCO portal to begin prototype manufacturing.
Engage with Approved Testing Labs: Schedule product testing early to avoid delays.
Compile Required Documentation: Prioritize preparation of Device Master File, Plant Master File, and Risk Management File.
Select a Notified Body for Audit: Refer to the official list of notified bodies to identify an auditor in your region.
Submit MD5 Application (Form MD3): Upload all documents and await audit scheduling.
Address Audit Findings and Queries: Respond promptly to expedite license grant.
Starting your CDSCO licensing journey with a clear plan and expert support ensures compliance and faster market access. Contact us to leverage our proven expertise in medical device regulatory approvals.
By following this comprehensive roadmap tailored for the Chorionic Villus Sampling Catheter, manufacturers and importers can confidently navigate the CDSCO licensing process, ensuring timely approval and successful entry into the Indian medical device market.