CDSCO License for Contact Lens (Including Coloured Contact Lens)
Medical Device Information
Intended Use
Device intended to be worn directly against the cornea and adjacent limbal and scleral areas of the eye to correct vision conditions or act as a therapeutic bandage or/and to change the appearance of the eye for decorative purposes.
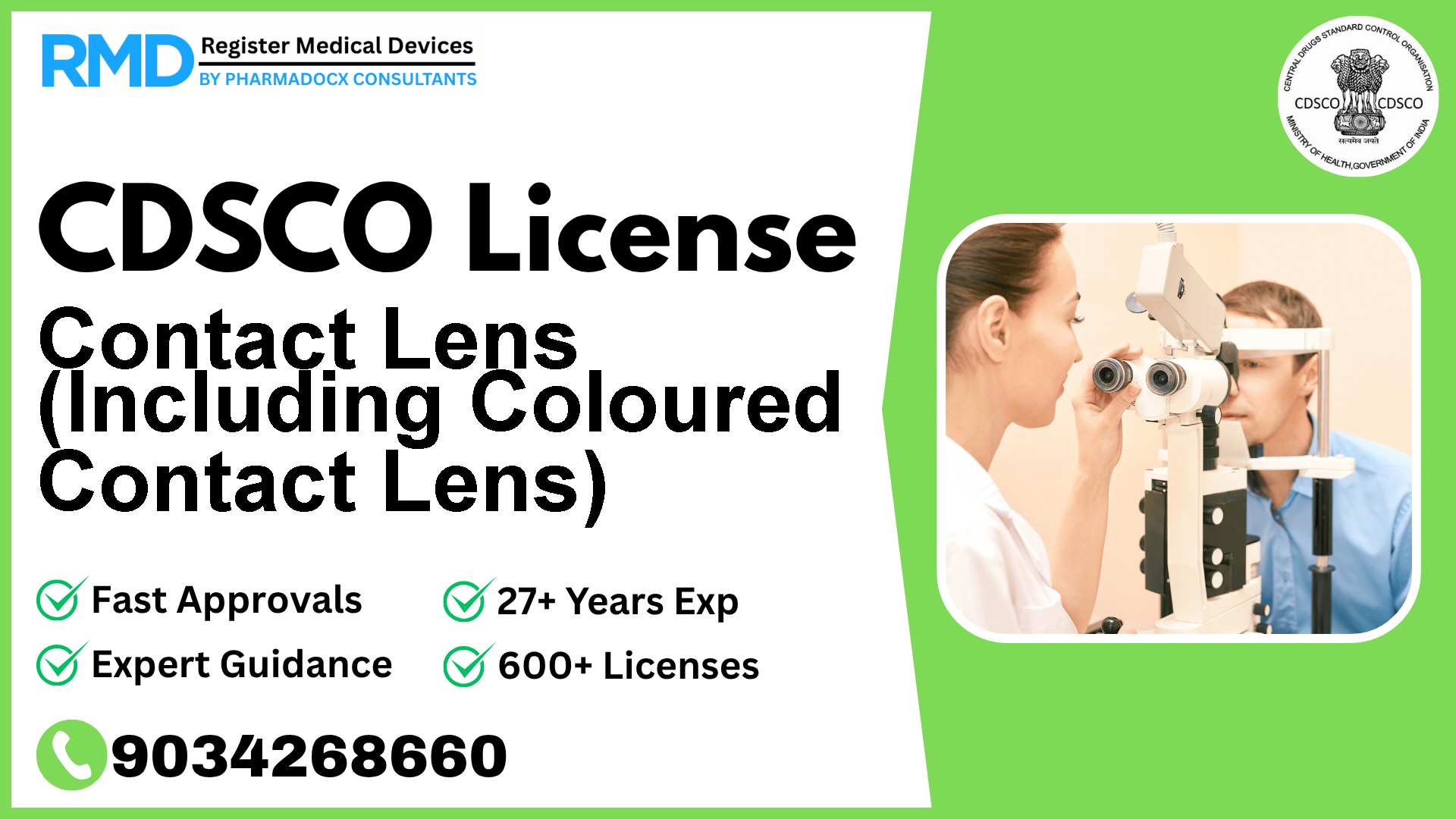
Comprehensive CDSCO Licensing Guide for Contact Lenses (Class B Medical Device)
Contact lenses, including coloured variants, are widely used ophthalmic devices intended to be worn directly against the cornea and adjacent ocular areas to correct vision, provide therapeutic benefits, or enhance eye appearance. Given their direct contact with sensitive eye tissue, these devices are classified as Class B under the Indian medical device regulatory framework. Obtaining a CDSCO manufacturing or import license is mandatory for legal marketing and distribution in India.
With over 25 years of experience and having successfully guided 500+ companies through the CDSCO licensing process, we provide you with an actionable, step-by-step overview tailored specifically for Contact Lens manufacturers and importers.
CDSCO Regulatory Framework for Contact Lenses
The regulatory oversight for contact lenses in India falls under the Central Drugs Standard Control Organization (CDSCO), which enforces compliance with the Medical Device Rules 2017. The notification specific to contact lenses is Fts No. 29/MiscJO3/2020-DC (187), dated 9th August 2021, officially recognizing contact lenses as Class B medical devices.
Manufacturers and importers must secure the appropriate manufacturing or import license before marketing these devices. Licenses are issued based on risk classification:
- Class A & B devices: Licensing by State Authorities (MD5 License)
- Class C & D devices: Licensing by Central Authorities (MD9 License)
- Imports: Licensing by Central Authorities (MD15 License)
Contact lenses fall under Class B, requiring an MD5 manufacturing license if produced domestically and an MD15 import license for foreign manufacturers or importers.
Risk Classification and License Requirements for Contact Lenses
Contact lenses are classified as Class B devices due to their moderate risk profile — they are invasive devices intended for short-term use in direct contact with the eye, but not implanted.
License Types:
- MD5 License (Form MD3): For manufacturing Class B devices within India, issued by the State Licensing Authority.
- MD15 License (Form MD14): For importing contact lenses, issued by the Central Licensing Authority.
Our detailed guides on Medical Device Classification and MD5 License can help clarify requirements further.
Manufacturing License Process for Contact Lenses (MD5 License)
The process to obtain an MD5 license for manufacturing contact lenses involves several key steps:
Test License Acquisition (Form MD13): Apply for a test license valid for 6 months to produce samples for testing. This typically takes 1.5 to 2 months.
Product Testing: Conduct mandatory product testing at government-approved laboratories. Find the list of Testing Laboratories authorized by CDSCO.
Document Preparation: Prepare essential documents including Device Master File, Plant Master File, Risk Management File, and others.
Application Submission: Submit the manufacturing license application on the CDSCO MD Online Portal using Form MD3.
Audit by Notified Body: Undergo an audit conducted by a notified body certified for Class B devices. Verify the list of notified bodies for audit requirements.
Query Resolution: Address any queries or observations raised by the licensing authority or notified body.
License Grant: Upon satisfactory completion, the MD5 manufacturing license is granted.
Timeline:
- Total process duration is approximately 3 to 4 months, including test license issuance, product testing, audit, and license grant.
Costs:
- Government fees: ₹5,000 per application plus ₹500 per product.
Practical Tip:
Start preparing your Device Master File early. Our Device Master File guide offers detailed templates and best practices tailored for ophthalmic devices.
Manufacturing License Documents Required for Contact Lenses
To ensure smooth processing, prepare the following key documents:
- Company Constitution (Incorporation Certificate, Memorandum & Articles of Association)
- Proof of ownership or lease agreement of manufacturing premises
- Technical staff qualifications and experience
- Fire No Objection Certificate (NOC)
- Pollution Control Board NOC (if applicable)
- Device Master File (DMF) detailing design, materials, manufacturing process
- Plant Master File (PMF) demonstrating facility compliance and quality systems
- Essential Principles Checklist confirming compliance with Indian standards
- Risk Management File documenting hazard analysis and mitigation strategies
- Product Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU) consistent with regulatory requirements
- Quality Management System (QMS) documentation, preferably ISO 13485 certified
Thorough documentation significantly reduces audit findings and query cycles.
Import License Process for Contact Lenses (MD15 License)
For importers of contact lenses, the MD15 import license process is centrally governed and includes:
Document Compilation: Collect all required certifications including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, and Device/Plant Master Files.
Application Submission: Submit the application using Form MD14 on the CDSCO MD Online Portal.
Review and Queries: The CDSCO Central Licensing Authority reviews the application and may raise clarifications.
License Issuance: Upon satisfactory review, the MD15 import license is granted.
Timeline:
- The entire import license process typically takes 5 to 6 months.
Costs:
- For Class B devices like contact lenses, government fees include:
- ₹2000 per site
- ₹1000 per product
Practical Insight:
Unlike the manufacturing process, no test license is required for imports, but product conformity documents must be rigorously prepared.
Explore our detailed Import License guide for additional nuances.
Timeline and Processing Duration Summary
| License Type | Steps Involved | Approximate Duration |
|---|---|---|
| MD5 Manufacturing | Test license, testing, audit | 3 - 4 months |
| MD15 Import | Document review, queries | 5 - 6 months |
Planning your project timeline around these durations helps avoid costly delays.
Government Fees and Costs
| License Type | Application Fee | Per Product Fee |
|---|---|---|
| MD5 (Class B) | ₹5,000 | ₹500 |
| MD15 Import (Class B) | ₹2,000 | ₹1,000 |
Additional costs to budget for include testing fees at government labs and notified body audit charges, which vary depending on scope and device complexity.
Common Challenges and Solutions
Delayed Product Testing: Testing labs may have backlogs. We recommend early booking and choosing labs with proven turnaround times.
Incomplete Documentation: Missing or inconsistent Device Master Files and Risk Management documentation cause query delays. Utilize expert templates and conduct internal audits before submission.
Audit Observations: Non-compliance with quality systems often leads to audit observations. Regular internal audits and staff training mitigate this risk.
Query Resolution Delays: Promptly addressing CDSCO queries with clear, documented responses expedites approvals.
Our extensive experience allows us to preempt such issues, ensuring smoother regulatory journeys.
Expert Consultation and Support
Navigating the complexities of CDSCO licensing for contact lenses requires in-depth knowledge of regulatory nuances, document preparation, and compliance strategies. We offer expert consulting services including:
- Customized gap analysis of your manufacturing/import setup
- End-to-end documentation assistance (DMF, PMF, Risk Management)
- Coordination with notified bodies and testing labs
- Query drafting and response management
- Post-license compliance and vigilance support
Trust our expertise to optimize your regulatory approval process – join the 500+ companies we've successfully served.
Getting Started with Your CDSCO License Application
Classify your device accurately as Class B and determine whether you require a manufacturing license (MD5) or import license (MD15).
Register on the CDSCO MD Online Portal to begin your application process.
Prepare your Test License application (Form MD13) if manufacturing, to commence product testing without delay.
Compile comprehensive technical documentation, prioritizing the Device Master File and Risk Management File.
Engage a notified body early to schedule your audit and align documentation accordingly.
Maintain open communication with CDSCO authorities, responding promptly to queries.
Leverage expert consulting for document reviews and process compliance.
Embarking on your CDSCO licensing journey with thorough preparation and expert support will significantly enhance your chances of timely approval and successful market entry for your contact lenses.
For additional resources, visit our MD5 License Guide, and learn more about Risk Management tailored for ophthalmic devices.
By following this comprehensive guide, manufacturers and importers of contact lenses can confidently navigate the CDSCO licensing landscape, ensuring compliance, minimizing delays, and successfully launching their products in the Indian market.