CDSCO License for Corneal burr manual instrument
Medical Device Information
Intended Use
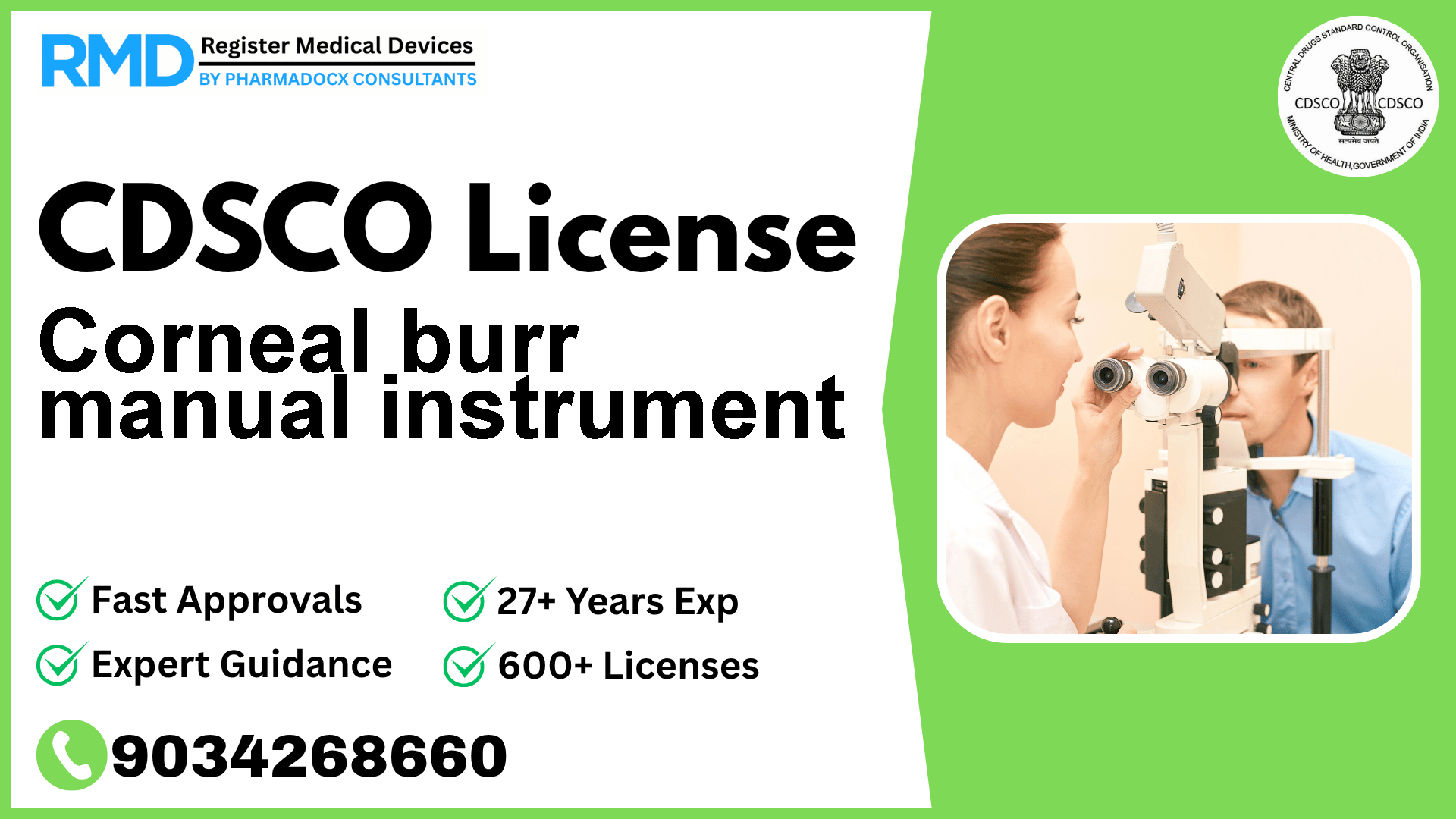
A hand-held, ophthalmic surgical instrument, used to excavate corneal tissue through manual rotation.
Comprehensive CDSCO Licensing Guide for Corneal Burr Manual Instrument (Class B Medical Device)
As seasoned regulatory consultants with over 25 years of experience and more than 500 successful CDSCO license approvals, we understand the challenges manufacturers and importers face in navigating India’s medical device regulations. The Corneal Burr Manual Instrument—a hand-held ophthalmic surgical tool used to manually excavate corneal tissue—is classified as a Class B device under the CDSCO framework. This classification mandates specific licensing, documentation, and compliance requirements that are critical to market access in India.
Understanding the CDSCO Regulatory Framework for Corneal Burr Manual Instruments
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India under the Medical Device Rules, 2017. The Corneal Burr falls under ophthalmic surgical instruments, notified under FTSC No. 29/MiscJO3/2020-DC (187) dated 9.8.2021. Given its Class B risk classification, the device requires a MD5 Manufacturing License issued by the State Licensing Authority.
This license ensures that the device meets safety, quality, and performance standards suitable for ophthalmic applications. Compliance with the Essential Principles Checklist, risk management, and quality management systems is mandatory.
Risk Classification and License Requirements for Corneal Burr
- Risk Class: B (Low to moderate risk)
- License Type: MD5 Manufacturing License (Application Form MD3)
- Issuing Authority: State Licensing Authority
- Applicable Rules: Medical Device Rules, 2017
You can verify device classification and regulatory requirements via our detailed Medical Device Classification guide.
Step-by-Step Manufacturing License Process for Corneal Burr (MD5)
- Obtain Test License (Form MD13): Before the manufacturing license application, the firm must apply for a test license valid for 1.5 to 2 months. This allows sample production for testing.
- Product Testing: Conduct mandatory testing in CDSCO-approved laboratories. Refer to the list of Testing Laboratories for accredited options.
- Document Preparation: Compile comprehensive technical and quality documents as per CDSCO requirements.
- Submit MD5 Application (Form MD3): Upload the application through the CDSCO MD Online Portal.
- Notified Body Audit: Engage an approved notified body for a manufacturing site audit. Consult the List of Notified Bodies.
- Query Resolution: Address any queries from the licensing authority or notified body promptly.
- License Grant: Upon satisfactory compliance, the State Licensing Authority issues the MD5 license.
Essential Documents Required for MD5 License Application
- Company Constitution Documents: Incorporation certificate, partnership deed, or equivalent
- Proof of Premises Ownership or Lease Agreement
- Technical Staff Qualifications: CVs and relevant certificates
- Fire NOC and Pollution Control NOC: From local authorities
- Device Master File (DMF): Detailed design, manufacturing process, and safety data (Device Master File Guide)
- Plant Master File (PMF): Facility layout, equipment details, and maintenance plans (Plant Master File Guide)
- Essential Principles Checklist: Compliance with Indian medical device standards
- Risk Management File: Hazard analysis and mitigation strategies (Risk Management Guide)
- Test Reports: From government-approved laboratories
- Labels and Instructions for Use (IFU): Device labeling and user manuals
- Quality Management System (QMS) Documents: ISO 13485:2016 certification and internal SOPs
Import License Process (MD15) for Corneal Burr
If you are an importer rather than a manufacturer, obtaining an MD15 license from the Central Licensing Authority is mandatory. The process includes:
- Document preparation including manufacturing license of the foreign manufacturer, Free Sale Certificate, ISO 13485:2016, CE Certificate
- Application submission on the CDSCO MD Online Portal
- Resolution of departmental queries
- License grant within approximately 5-6 months
Refer to our comprehensive Import License Guide for detailed assistance.
Timeline and Processing Duration
| Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 2 to 3 weeks |
| Document Preparation | 2 to 4 weeks |
| Application Submission & Audit | 1 to 2 months |
| Query Resolution & License Grant | 2 to 4 weeks |
Total estimated time: Approximately 3 to 4 months for the entire MD5 license process.
Government Fees and Associated Costs
- Test License (MD13): Approximately Rs. 2000 - Rs. 3000
- MD5 License Application Fee: Rs. 5000 per application
- Product Fee: Rs. 500 per product (per variant/model)
- Notified Body Audit Fees: Variable, typically Rs. 50,000 - Rs. 1,00,000 depending on the scope
- Testing Laboratory Charges: Rs. 20,000 - Rs. 50,000 depending on tests required
Budgeting realistically for these fees and the timeframes can help avoid last-minute disruptions.
Common Challenges and Practical Solutions
- Incomplete Documentation: Ensure all files—especially DMF, PMF, and risk management documents—are comprehensive and accurate. Use our guides on Device Master Files and Plant Master Files to streamline preparation.
- Delayed Testing: Plan product testing early and select laboratories with shorter turnaround times.
- Audit Non-Compliance: Conduct internal mock audits before notified body visits to identify gaps.
- Query Response Delays: Maintain a dedicated regulatory contact for prompt communication with CDSCO.
Expert Consultation and Support
With our extensive experience assisting over 500 clients, we offer tailored support including:
- Gap analysis and readiness assessment
- Document drafting and review
- Coordination with notified bodies and testing labs
- Application submission and tracking
- Post-license regulatory compliance training
Our hands-on approach ensures that your Corneal Burr manufacturing or import license application progresses smoothly and efficiently.
Getting Started with Your CDSCO License Application for Corneal Burr
- Assess your facility and technical team readiness. Confirm you have qualified personnel and compliant manufacturing premises.
- Initiate the Test License application (Form MD13) via the CDSCO MD Online Portal.
- Schedule product testing early with approved laboratories.
- Start compiling your Device Master File and Plant Master File leveraging expert templates to avoid common pitfalls.
- Engage a notified body for pre-audit consultation to preempt any non-conformities.
- Submit your MD5 application (Form MD3) once testing and documentation are ready.
- Prepare for audit and promptly address any queries.
Embarking on this structured regulatory pathway with expert guidance will maximize your chances of a timely and successful CDSCO license grant for your Corneal Burr Manual Instrument. Connect with us today to leverage our proven expertise and industry insights to bring your ophthalmic device to the Indian market confidently.