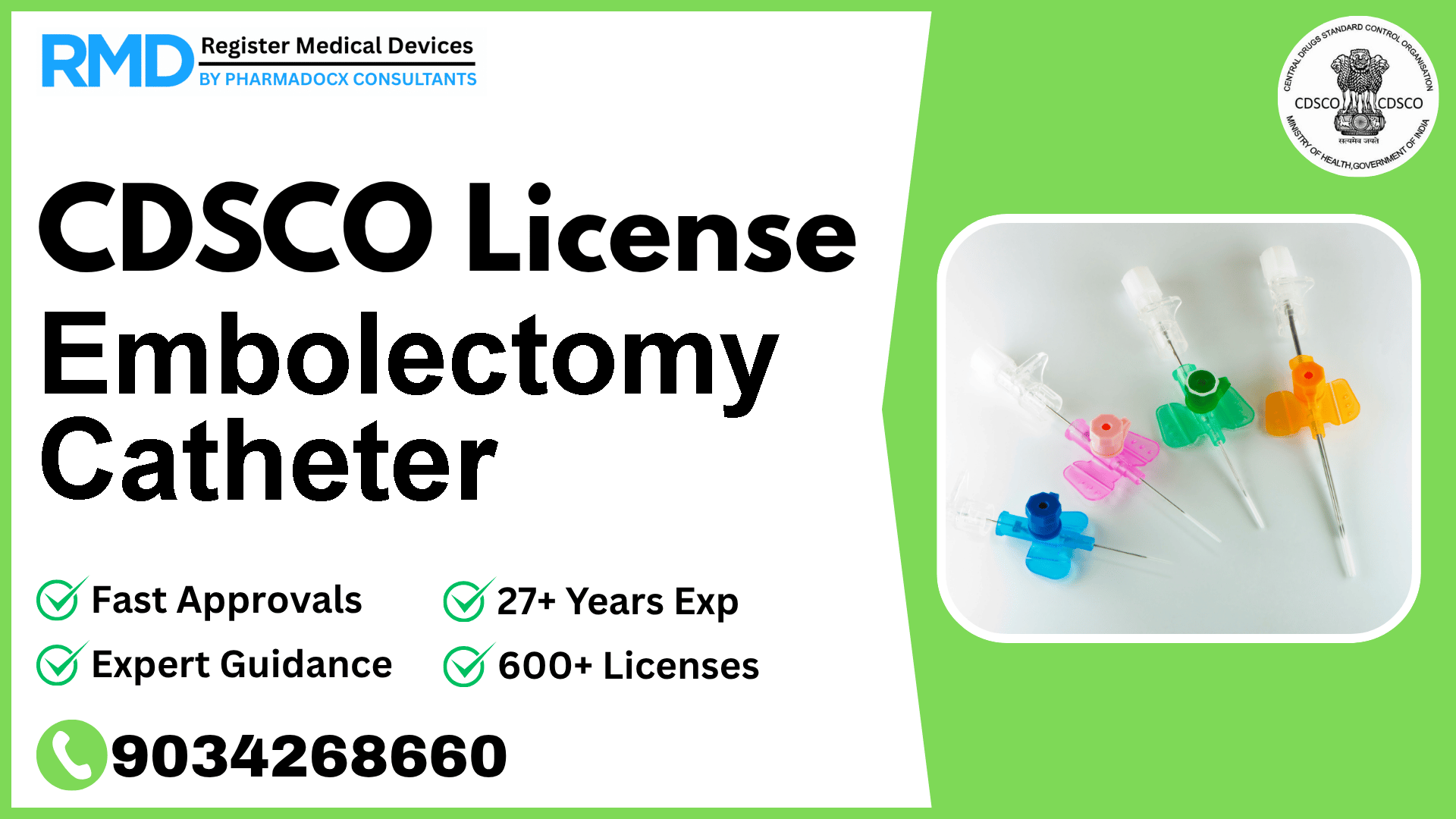
CDSCO License for Embolectomy Catheter
Medical Device Information
Intended Use
indicated for the removal of fresh, soft emboli and
Comprehensive Guide to CDSCO Licensing for Embolectomy Catheters (Class D Medical Device)
Embolectomy catheters are critical medical devices designed specifically for the removal of fresh, soft emboli from blood vessels, playing a vital role in acute thromboembolic events. Given their high-risk classification (Class D) under the Indian regulatory framework, obtaining a CDSCO manufacturing or import license is a rigorous but essential process to ensure patient safety and compliance with Indian laws. With over 25 years of regulatory consulting experience and having supported more than 500 companies, we provide you with an expert roadmap tailored for embolectomy catheters to navigate the CDSCO licensing landscape efficiently.
CDSCO Regulatory Framework for Embolectomy Catheters
The Central Drugs Standard Control Organization (CDSCO) is the apex regulatory body governing medical devices in India. The Embolectomy Catheter falls under the category of catheters and is notified under the Gazette Notification 29/Misc/3/2017-DC (292) dated 06.06.2018. As a Class D device, it is subject to strict oversight due to its potential impact on human health.
Risk Classification and License Requirements for Embolectomy Catheters
- Risk Class: D (Highest risk category)
- Regulatory Authority: Central Licensing Authority (CLA)
- Applicable License: MD9 Manufacturing License for manufacturers and MD15 Import License for importers
Class D devices like embolectomy catheters require thorough conformity assessment, including product testing, quality system audits, and detailed documentation to demonstrate compliance with safety and performance standards.
Manufacturing License Process (MD9) for Embolectomy Catheters
The manufacturing license for Class D devices is issued through the MD9 license process (Form MD7 application). This process involves several key steps:
- Test License (Form MD13): Initially, obtain a test license allowing production of samples for testing. This stage typically takes 1.5 to 2 months.
- Product Testing: Conduct mandatory product testing at CDSCO-approved laboratories to verify compliance with Indian standards. You can find an updated list of government-approved testing laboratories on the CDSCO portal.
- Document Preparation: Prepare comprehensive documentation including Device Master File (DMF), Plant Master File (PMF), Essential Principles Checklist, Risk Management File, and Quality Management System (QMS) documentation.
- Application Submission: Apply for the MD9 manufacturing license through the CDSCO MD Online Portal using Form MD7.
- Inspection and Audit: CDSCO inspectors conduct a detailed audit of your manufacturing facility and QMS compliance.
- Query Resolution: Address any queries raised by CDSCO inspectors promptly.
- License Grant: Upon successful compliance, the MD9 license is granted.
Manufacturing License Documents Required
For a Class D device like the Embolectomy Catheter, prepare the following documents meticulously:
- Company Constitution (Incorporation Certificate, Partnership Deed, etc.)
- Proof of ownership or lease agreement of manufacturing premises
- Qualification and experience details of technical staff
- Fire No Objection Certificate (Fire NOC)
- Pollution Control Board NOC
- Device Master File (DMF): Detailed device design, specifications, manufacturing processes, and validation reports (learn more)
- Plant Master File (PMF): Details of manufacturing site, equipment, utilities, and quality control systems (guide here)
- Essential Principles Checklist confirming compliance with Indian medical device rules
- Risk Management File following ISO 14971 standards (risk management insights)
- Test Reports from CDSCO-approved laboratories
- Product labels and Instructions for Use (IFU) compliant with regulatory requirements
- Quality Management System Documents (ISO 13485:2016 certification is highly recommended)
Import License Process (MD15) for Embolectomy Catheters
Importers of embolectomy catheters must apply for the MD15 import license using Form MD14. The process differs from manufacturing licensing in that a test license is not required, but documentation rigor and approvals from central authorities remain stringent.
Key steps include:
- Document Compilation: Prepare import license application with all required certificates and compliance documents.
- Application Submission: Submit via the CDSCO MD Online Portal using Form MD14.
- Query Resolution: Respond to any departmental queries.
- License Issuance: Receive the import license (MD15) upon approval.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate or Certificate of Market Authorization
- ISO 13485:2016 certification
- CE Certificate or equivalent international certification
- Device Master File (DMF)
- Plant Master File (PMF)
- Wholesale license (if applicable)
- Company Constitution and legal documents
Timeline and Processing Duration
| License Type | Process Duration |
|---|---|
| MD9 Manufacturing | 4 to 5 months (including test license, testing, audit) |
| MD15 Import | 5 to 6 months |
The MD9 process begins with a 1.5 to 2-month test license period, followed by product testing and document submission. Audits and query resolution may extend the timeline but adhering to documentation completeness can help reduce delays.
Government Fees and Costs
| License Type | Application Fee | Per Product Fee |
|---|---|---|
| MD9 (Class D) | ₹50,000 | ₹1,000 |
| MD15 (Import) | ₹3,000 per site | ₹1,500 per product |
Note: Additional costs include testing fees at government-approved labs, notified body audit fees, and consultancy charges if applicable.
Common Challenges and Solutions
- Incomplete Documentation: Many applicants underestimate the importance of comprehensive Device and Plant Master Files. Our detailed guides ensure your documentation is audit-ready.
- Testing Delays: Booking slots at CDSCO-approved labs can be competitive. Early scheduling and pre-audit internal testing help mitigate delays.
- Audit Non-Compliance: Lack of preparedness for technical audits can result in repeated inspections. We recommend mock audits and staff training.
- Query Management: Delayed or inadequate responses to CDSCO queries prolong licensing. Maintaining an organized query tracking system is crucial.
Expert Consultation and Support
Navigating the CDSCO licensing process for high-risk devices like the Embolectomy Catheter requires expert insight. Our team has assisted over 500 companies in successfully obtaining MD9 and MD15 licenses. We offer:
- End-to-end application preparation and submission
- Comprehensive documentation support including DMF and PMF
- Coordinating product testing and audit readiness
- Query resolution and liaison with CDSCO authorities
Getting Started with Your CDSCO License Application
If you are planning to manufacture or import embolectomy catheters in India, here are your practical next steps:
- Classify Your Device: Confirm the Class D status and understand your obligations by reviewing the medical device classification.
- Prepare Test License Application (for manufacturers): Apply for the MD13 test license to begin sample production.
- Identify Testing Labs: Select a CDSCO-approved testing laboratory early to avoid bottlenecks (approved labs here).
- Develop Documentation: Start compiling your Device Master File and Plant Master File using our comprehensive guides.
- Engage Notified Bodies for Audit: Familiarize with the list of notified bodies authorized to conduct audits.
- Submit Application via CDSCO Portal: Use the CDSCO MD Online Portal for all submissions.
- Plan for QMS Certification: ISO 13485:2016 certification can streamline your audit and licensing process.
Our proven approach and thorough support can dramatically improve your chances of a smooth and timely CDSCO license approval. Contact us to leverage our 25+ years of expertise and tailored consulting services.
By following this detailed roadmap and utilizing our expert guidance, manufacturers and importers of embolectomy catheters can confidently enter the Indian medical device market in full compliance with CDSCO regulations.