CDSCO License for Eye cup
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A receptacle designed to fit around the eye socket and which is filled with warm water or an eyewash solution and placed over the eye to allow the liquid to wash the affected eye.
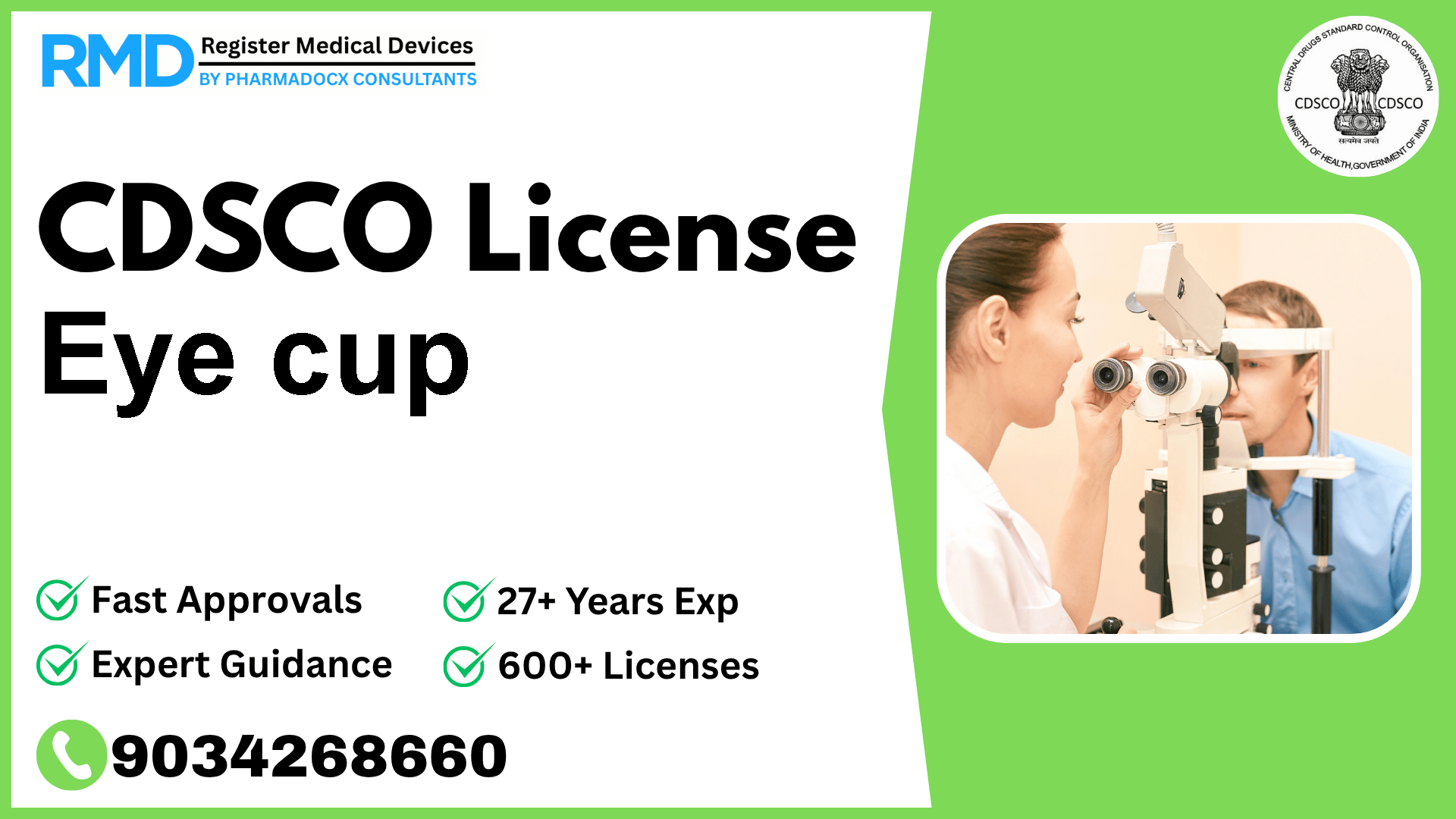
Introduction to Eye Cup and Its Regulatory Importance
Eye cups are essential ophthalmic devices designed as a receptacle fitting around the eye socket, filled with warm water or eyewash solution to cleanse the affected eye. Given their medical application, regulatory compliance is crucial to ensure safety, quality, and market access in India. With the notification Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021, the Eye Cup is classified as a Class A medical device under the ophthalmology category, necessitating a CDSCO license before manufacturing or import.
At our consultancy, with over 25 years of experience and having assisted 500+ companies, we understand the nuances and practical challenges manufacturers and importers face. This guide offers an end-to-end overview of the CDSCO licensing process specifically for Eye Cups, helping you navigate the regulatory pathway efficiently.
CDSCO Regulatory Framework for Eye Cups
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India under the Medical Devices Rules, 2017. Eye Cups fall under Class A (low risk) devices. The licensing process is governed by the State Licensing Authority for manufacturing and the Central Licensing Authority for imports.
Compliance involves adherence to the Essential Principles of Safety and Performance, quality management systems, and submission of appropriate documentation including Device Master Files and Plant Master Files.
Risk Classification and License Requirements for Eye Cups
Eye Cups are categorized as Class A devices, the lowest risk class under CDSCO regulations. This classification means:
- Manufacturing License: MD5 License (Application Form MD3) obtained from the State Licensing Authority.
- Import License: N/A for manufacturing but if importing, MD15 License from the Central Licensing Authority.
Class A devices require a test license (MD13) before full manufacturing license issuance, product testing from government-approved labs, and audit by notified bodies.
Learn more about medical device classification on our Medical Device Classification resource.
Manufacturing License Process for Eye Cups (MD5)
For manufacturers intending to produce Eye Cups in India, the licensing process follows these steps:
- Test License Application (Form MD13): Apply for a test license to manufacture on a trial basis. Processing typically takes 1.5 to 2 months.
- Product Testing: Get Eye Cups tested at CDSCO-approved laboratories. Refer to the Testing Laboratories list.
- Document Preparation: Compile all required documents including Device Master File, Plant Master File, Essential Principles Checklist, and Risk Management File.
- Manufacturing License Application (Form MD3): Submit the application for MD5 license via the CDSCO MD Online Portal.
- Audit by Notified Body: An external audit is conducted to verify compliance. Check the list of notified bodies for audit assignments.
- Query Resolution: Address any queries raised by authorities or auditors promptly.
- License Grant: Upon satisfactory compliance, the MD5 license is granted.
Manufacturing License Documents Required for Eye Cups
Ensuring complete and accurate documentation accelerates approvals. For Eye Cups (Class A), the required documents include:
- Company Constitution and Incorporation Documents
- Proof of Ownership or Tenancy of Manufacturing Premises
- Qualification and Experience Certificates of Technical Staff
- Fire Safety and Pollution Control NOCs
- Device Master File (DMF) detailing design, specifications, and manufacturing processes. Our comprehensive Device Master File guide can assist you.
- Plant Master File (PMF) describing manufacturing facilities and quality controls. Learn how to create an efficient PMF here.
- Essential Principles Checklist confirming compliance with safety and performance standards
- Risk Management File demonstrating risk assessments aligned with ISO 14971
- Product Test Reports from CDSCO-approved labs
- Labeling and Instructions for Use (IFU) documents
- Quality Management System (QMS) documents, typically ISO 13485 certification
Import License Process for Eye Cups (MD15)
If you plan to import Eye Cups into India, you must apply for an MD15 import license with the Central Licensing Authority. The process is as follows:
- Document Preparation: Gather necessary documents including manufacturing license of the foreign manufacturer, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device Master File, Plant Master File, wholesale license, and company constitution.
- Application Submission: File Form MD14 via the CDSCO MD Online Portal.
- Query Resolution: Respond to department queries promptly.
- License Issuance: After review, the MD15 import license is granted.
Import License Documents Required
Key documents for import license include:
- Manufacturing License of foreign manufacturer
- Free Sale Certificate from country of origin
- ISO 13485:2016 certificate
- CE Certificate or equivalent
- Device Master File and Plant Master File
- Wholesale License for distribution in India
- Company Constitution and Incorporation Documents
Timeline and Processing Duration
Manufacturing License (MD5) for Eye Cups:
- Test License (MD13): 1.5 to 2 months
- Product Testing: 3 to 4 weeks
- Document Preparation and Submission: 2 to 3 weeks
- Audit by Notified Body: 3 to 4 weeks
- Query Resolution and Final Approval: 2 to 3 weeks
Total estimated duration: 3 to 4 months
Import License (MD15): Approximately 5 to 6 months
Government Fees and Costs
For Class A Eye Cups, the fee structure is:
- Test License (MD13): Rs. 5,000
- Manufacturing License (MD5): Rs. 5,000 per application + Rs. 500 per product
Additional costs include:
- Product testing charges at government-approved labs (varies by test scope)
- Audit fees payable to the notified body
- Consultancy fees (if availed)
Planning your budget upfront ensures smooth financial management.
Common Challenges and Solutions
Challenge: Delays in test license approval or product testing.
- Solution: Submit complete applications with all required documents, select CDSCO-approved labs with shorter turnaround times.
Challenge: Incomplete or inconsistent documentation leading to audit non-compliance.
- Solution: Use comprehensive templates for Device Master File and Plant Master File; conduct internal audits before official audits.
Challenge: Responding to multiple queries from authorities.
- Solution: Maintain a dedicated regulatory team or expert consultancy support for prompt and accurate responses.
Challenge: Uncertainty about notified body selection.
- Solution: Refer to the official notified bodies list and choose organizations with relevant ophthalmic device expertise.
Expert Consultation and Support
Navigating CDSCO licensing for Eye Cups can be complex, especially for first-time applicants. Our team, with 25+ years of regulatory expertise, has successfully guided over 500 companies through the entire process—from test license to final approval. We assist with document preparation, audit readiness, product testing coordination, and timely query resolution.
Partnering with experienced consultants reduces risks of delays and increases your chances of first-time approval.
Getting Started with Your CDSCO License Application
To initiate your CDSCO licensing journey for Eye Cups:
- Assess your product classification and regulatory requirements. Confirm Class A designation and applicable license types.
- Prepare your Device Master File and Plant Master File meticulously. Utilize our detailed DMF guide and PMF guide for best practices.
- Apply for the Test License (MD13) via the CDSCO MD Online Portal.
- Coordinate product testing at CDSCO-approved labs to meet quality standards.
- Schedule and prepare for the notified body audit to ensure compliance.
- Submit your manufacturing license application (Form MD3) after successful test license and audit.
By following these steps with expert guidance, you can efficiently obtain your CDSCO MD5 license and bring your Eye Cups to the Indian market with confidence.
For personalized assistance or to discuss your specific project, contact us today and leverage our proven regulatory strategies for success.