CDSCO License for Fornixscope
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A manually-operated, ophthalmic device intended to provide indirect access and viewing of the upper conjunctival fornix and inner surface of the eyelid as an alternative to eyelid eversion.
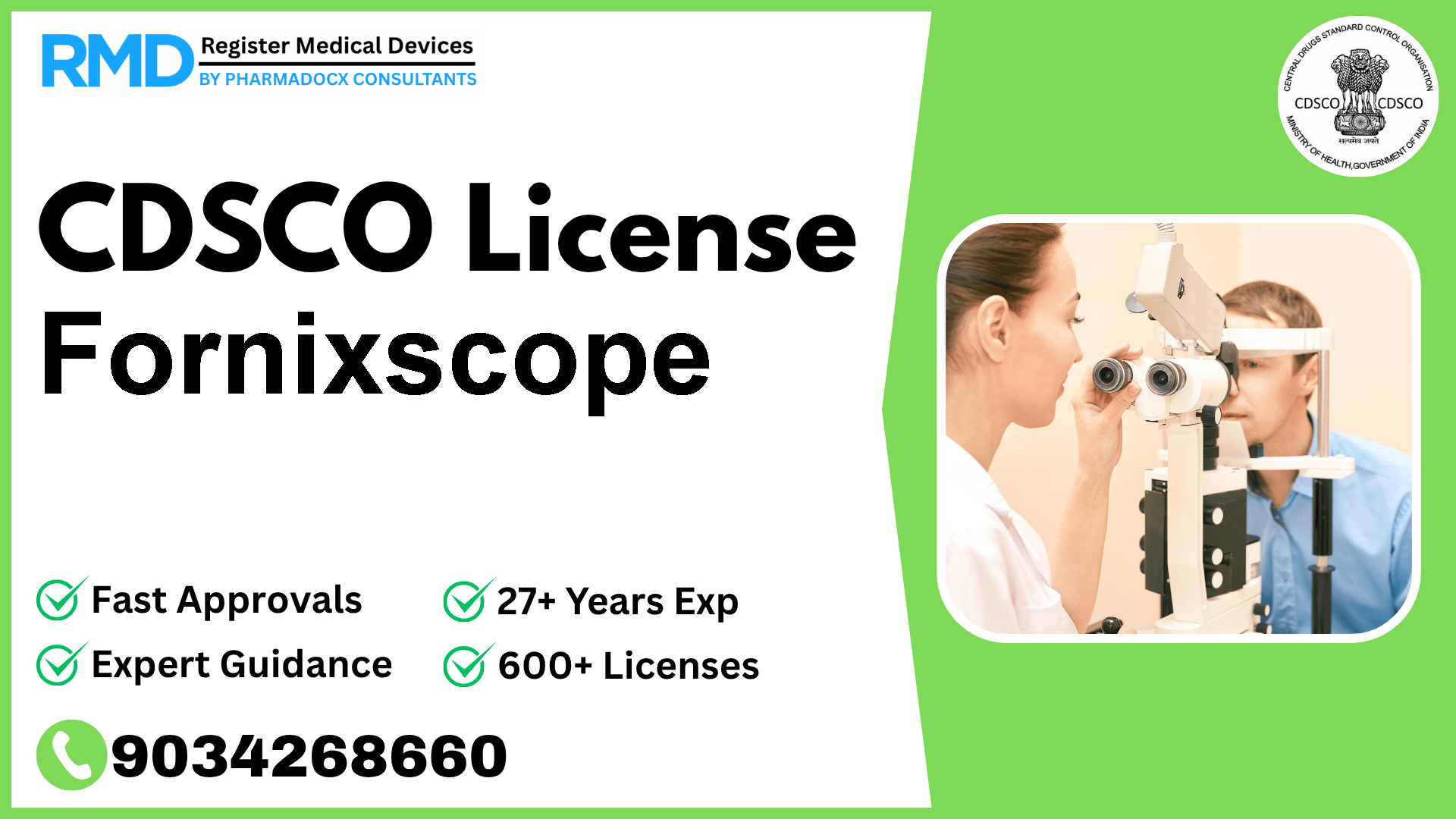
Comprehensive Guide to CDSCO Licensing for Fornixscope: Class A Ophthalmic Device
The Fornixscope, a manually-operated ophthalmic instrument designed to provide indirect access and visualization of the upper conjunctival fornix and inner surface of the eyelid, represents an innovative alternative to traditional eyelid eversion. Given its critical role in ophthalmology, obtaining proper regulatory approval is essential to ensure patient safety and market access in India.
With over 25 years of experience guiding more than 500 medical device manufacturers and importers, we provide an expert roadmap to securing your CDSCO license for the Fornixscope, categorized as a Class A medical device.
Understanding the CDSCO Regulatory Framework for Ophthalmic Devices like Fornixscope
The Central Drugs Standard Control Organization (CDSCO) governs medical device regulation in India under the Medical Device Rules, 2017. The Fornixscope falls under the ophthalmology category and is classified as Class A, the lowest risk classification, subject to State Licensing Authority oversight.
The device is notified under Fts No. 29/MiscJO3/2020-DC (187), dated 9.8.2021, making compliance with CDSCO regulations mandatory for manufacturing and marketing within India.
Risk Classification and License Requirements for Fornixscope
Being a Class A device, the Fornixscope requires an MD5 manufacturing license issued by the respective State Licensing Authority. This license ensures adherence to safety, quality, and performance standards as per the Medical Device Rules.
Key highlights:
- Risk Class: A (Low Risk)
- License Type: MD5 (Manufacturing License for Class A & B devices)
- Regulatory Body: State Licensing Authority
MD5 Manufacturing License Process for Fornixscope
The MD5 license process is comprehensive yet streamlined for Class A devices. The steps include:
Obtain Test License (Form MD13): Initiate with a test license application to produce limited product batches for testing. This phase takes approximately 1.5 to 2 months.
Compliance Testing: Get the Fornixscope tested at CDSCO-approved laboratories to validate performance and safety. Refer to the list of testing laboratories for accredited centers.
Documentation Preparation: Develop thorough documentation including Device Master File, Plant Master File, Quality Management System (QMS) documents, Risk Management File, and Essential Principles Checklist.
Application Submission (Form MD3): Apply for the MD5 manufacturing license via the CDSCO MD Online Portal.
Audit by Notified Body: Undergo an audit by a notified body accredited for Class A devices. Check the notified bodies list to select an appropriate auditor.
Query Resolution: Address any queries or clarifications raised by the licensing authority or notified body.
License Grant (Form MD5): Upon satisfactory compliance, the State Licensing Authority grants the MD5 manufacturing license.
Documents Required for MD5 Manufacturing License Application
For the Fornixscope application, the following detailed documents are essential:
- Company Constitution (Incorporation Certificate, Partnership Deed, etc.)
- Proof of Ownership or Lease Agreement for Manufacturing Premises
- Qualification and Experience Details of Technical Staff
- Fire Safety and Pollution Control NOCs
- Device Master File detailing design, development, and manufacturing processes. Our comprehensive Device Master File guide can assist.
- Plant Master File outlining facility details and equipment. Learn more from our Plant Master File Guide.
- Essential Principles Checklist demonstrating compliance with safety standards
- Risk Management File specific to the Fornixscope
- Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documents (ISO 13485:2016 certification recommended)
Import License Process for Fornixscope (If Applicable)
For importers intending to bring the Fornixscope into India, an MD15 import license from the Central Licensing Authority is mandatory. This process generally takes 5 to 6 months and includes:
- Preparation of documents (including Manufacturing License from country of origin, Free Sale Certificate, ISO 13485:2016, CE certificate)
- Submission of application via CDSCO MD Online Portal
- Resolution of queries raised by CDSCO
- Issuance of MD15 license
For a detailed walkthrough, you may refer to our Import License Guide.
Timeline and Processing Duration
For the Fornixscope MD5 manufacturing license:
- Test License (MD13): 1.5 - 2 months
- Product Testing: 1 - 1.5 months
- Application Processing and Audit: 1.5 - 2 months
- Total Expected Duration: Approximately 3 to 4 months
Timely preparation of documents and proactive resolution of queries can significantly reduce delays.
Government Fees and Costs
The fee structure for MD5 license of Class A devices is as follows:
- Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product (Fornixscope considered as one product)
Additional costs include testing fees at government-approved labs and audit charges by notified bodies.
Common Challenges and Practical Solutions
Challenge: Delays in Testing and Audit Scheduling
- Solution: Early coordination with notified bodies and testing labs; book slots well in advance.
Challenge: Incomplete or Inconsistent Documentation
- Solution: Use templates and checklists for Device Master File and Risk Management File. Consult experts for document review.
Challenge: Query Resolution Delays
- Solution: Assign a dedicated regulatory liaison to respond promptly to CDSCO queries.
Expert Consultation and Support
Navigating the CDSCO regulatory landscape for ophthalmic devices like the Fornixscope requires specialized expertise. Our team has supported over 500 companies in achieving successful license approvals. We offer:
- End-to-end license application management
- Document preparation and review
- Coordination with notified bodies and testing laboratories
- Training on regulatory compliance and post-market surveillance
Getting Started with Your CDSCO License Application for Fornixscope
- Assess Your Readiness: Conduct an internal audit of your manufacturing setup and documentation.
- Engage with Testing Labs: Identify and schedule testing at CDSCO-approved laboratories.
- Prepare Documentation: Utilize our detailed guides on Device and Plant Master Files.
- Apply for Test License: Submit Form MD13 via the CDSCO MD Online Portal.
- Plan for Audit: Select a notified body and prepare for the compliance audit.
Starting early and maintaining clear communication with regulatory authorities will streamline your journey to market.
For personalized support or to initiate your Fornixscope CDSCO licensing process, contact our regulatory experts today and leverage our 25+ years of proven success.