CDSCO License for Gastrostomy aspiration system stomach tube
Medical Device Information
Intended Use
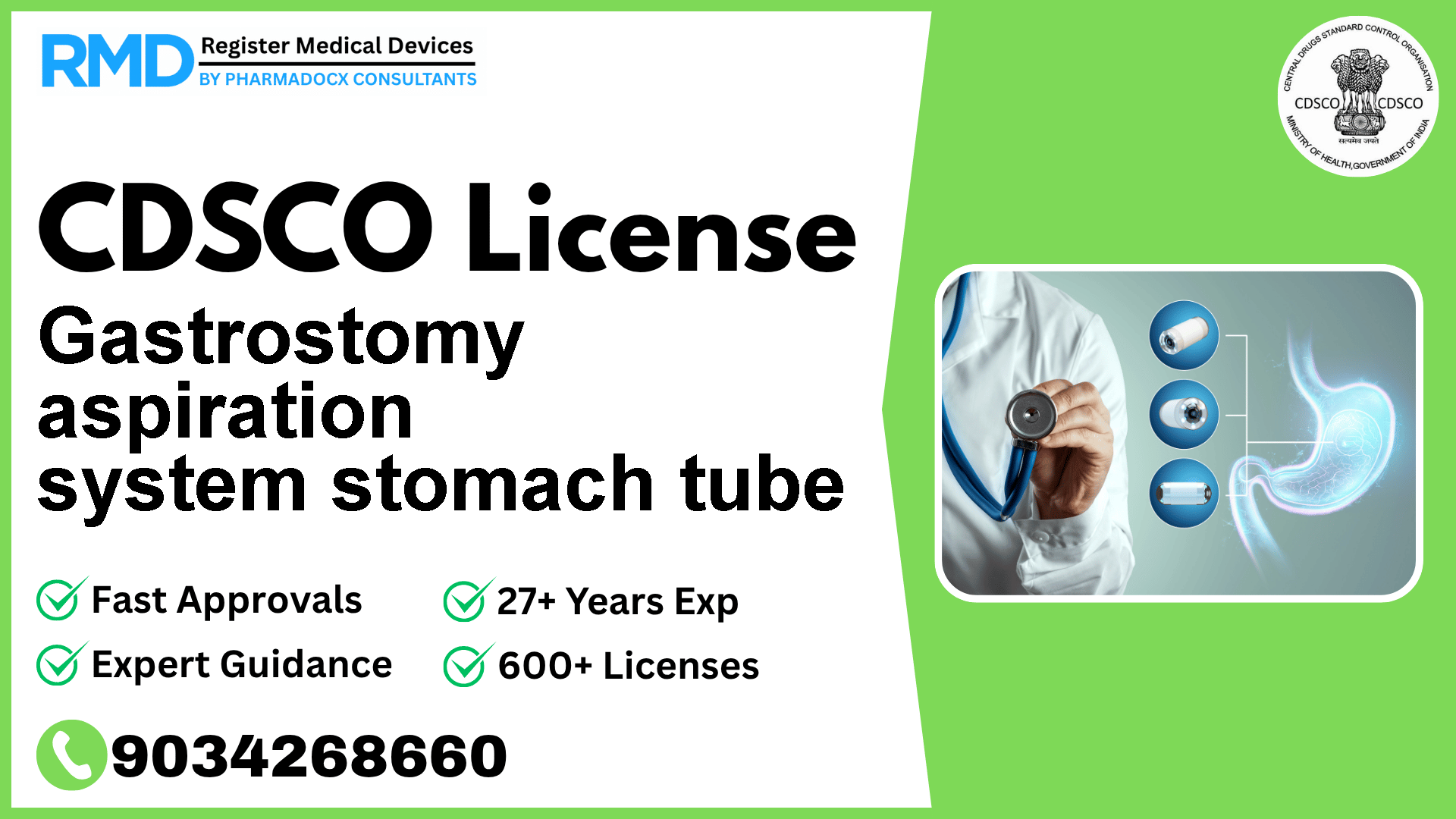
A sterile, thin, flexible, hollow cylinder intended to be percutaneously implanted by endoscopic methods into the stomach of a morbidly obese (bariatric) patient (≥ 18 years) for the removal of a portion of stomach contents after meals by aspiration when used with a dedicated gravity kit that is the external portion of a gastrostomy aspiration system.
Comprehensive Guide to CDSCO Licensing for Gastrostomy Aspiration System Stomach Tube (Class C Medical Device)
As a medical device regulatory consultancy with over 25 years of experience, having supported 500+ manufacturers and importers in India, we understand the complexities involved in securing CDSCO licenses, especially for specialized devices like the Gastrostomy Aspiration System Stomach Tube. This Class C gastroenterology device demands strict compliance with regulatory norms to ensure safety, efficacy, and market access.
Understanding the Device and Regulatory Importance
The Gastrostomy Aspiration System Stomach Tube is a sterile, flexible, hollow tube designed for percutaneous endoscopic implantation in bariatric patients to aspirate stomach contents post-meals. Classified as Class C due to its invasive nature and intended use, it falls under a higher risk category requiring rigorous regulatory scrutiny. Compliance with CDSCO regulations ensures patient safety and facilitates smooth market entry in India.
CDSCO Regulatory Framework for Gastrostomy Aspiration System Stomach Tube
The Central Drugs Standard Control Organisation (CDSCO) regulates medical devices under the Medical Device Rules, 2017. Devices are classified into four risk classes (A to D). Being a Class C device, the Gastrostomy Aspiration System requires a manufacturing license (MD9) issued by the Central Licensing Authority. Importers also need relevant approvals for market entry.
Risk Classification and License Requirements
- Risk Class: C
- Regulatory Pathway: MD9 Manufacturing License
- Application Form: MD7
- Authority: Central Licensing Authority (CDSCO HQ)
- Process includes: Test license (MD13), product testing, audit, and final license grant
For detailed risk classification, manufacturers can refer to the Medical Device Classification guide.
MD9 Manufacturing License Process for Class C Devices
- Test License Application (Form MD13): Initiate by applying for a test license. It allows limited manufacturing for testing and validation.
- Product Testing: Conduct mandatory testing at government-approved laboratories to verify safety and performance. Refer to the Testing Laboratories list.
- Documentation Preparation: Prepare comprehensive technical dossiers including Device Master File (DMF), Plant Master File (PMF), Essential Principles Checklist, Risk Management File, and Quality Management System (QMS) documents.
- License Application (Form MD7): Submit the MD9 manufacturing license application through the CDSCO MD Online Portal.
- Audit: CDSCO inspectors conduct an on-site audit to verify compliance.
- Queries and Clarifications: Address any observations or queries raised during the audit.
- License Grant: Upon satisfactory compliance, the MD9 manufacturing license is issued.
For a detailed walkthrough, our MD9 License Guide offers practical insights.
Manufacturing License Documents Required for Gastrostomy Aspiration System
- Company Constitution (Incorporation Certificate, MOA/AOA)
- Proof of Ownership/Lease Agreement of Manufacturing Premises
- Details and qualifications of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design, specifications, and manufacturing process (DMF Guide)
- Plant Master File (PMF) describing manufacturing facilities and equipment (PMF Guide)
- Essential Principles Checklist confirming compliance with safety and performance standards
- Risk Management File documenting hazard identification and mitigation (Risk Management)
- Product Test Reports from CDSCO-approved labs
- Labels and Instructions for Use (IFU)
- Quality Management System documentation (e.g., ISO 13485:2016 certification)
Import License Process (MD15) for Gastrostomy Aspiration System
If you are an importer, you must obtain an import license (MD15) from CDSCO Central Licensing Authority:
- Application Form: MD14
- Documents Required: Manufacturing License from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate (if applicable), Device Master File, Plant Master File, Wholesale License, Company Constitution
- Fees: Class C devices attract USD 3000 per site and USD 1500 per product
- Timeline: Typically 5-6 months
Our Import License Guide can help streamline this process.
Timeline and Processing Duration
| Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 3 – 4 weeks |
| Documentation Prep | 2 – 3 weeks |
| MD9 Application Review | 1 – 2 months |
| Audit & Query Handling | 1 – 1.5 months |
| Total Time | 4 – 5 months |
Government Fees and Costs
- Test License (MD13): Nominal fee as per CDSCO norms
- MD9 Manufacturing License: ₹50,000 per application + ₹1,000 per product
- Product Testing: Varies depending on complexity; typically ₹50,000 - ₹1,00,000 per product
- Audit Fees: Included in license application fees
Common Challenges and Solutions
Challenge 1: Delays in Product Testing
- Solution: Engage accredited labs early and schedule tests proactively.
Challenge 2: Incomplete Documentation
- Solution: Use standardized templates for DMF, PMF, and risk files. Our detailed guides help ensure completeness.
Challenge 3: Audit Non-Compliance
- Solution: Conduct internal audits and mock inspections prior to CDSCO audit.
Challenge 4: Query Resolution Delays
- Solution: Assign dedicated regulatory personnel to respond promptly with clear, evidence-backed answers.
Expert Consultation and Support
Navigating CDSCO licensing for Class C devices like the Gastrostomy Aspiration System requires expertise. We provide end-to-end support—from gap analysis, documentation preparation, test lab coordination, to audit readiness—ensuring your application is robust and compliant. Our successful track record speaks volumes.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm Class C status with CDSCO guidelines.
- Prepare Technical Documentation: Start compiling Device and Plant Master Files.
- Apply for Test License (MD13): Submit test license application via the CDSCO MD Online Portal.
- Plan Product Testing: Coordinate with CDSCO-approved labs early.
- Schedule Internal Audits: Prepare your manufacturing facility and QMS.
- Submit MD9 License Application: Once test license and testing are complete, file your MD9 application (Form MD7).
- Engage with CDSCO and Notified Bodies: Respond to queries promptly and prepare for audits.
Embarking on this journey with seasoned consultants can drastically reduce your timelines and enhance approval success rates. Reach out to us for a personalized compliance strategy tailored to your Gastrostomy Aspiration System.
Leverage our in-depth knowledge and 25+ years of experience to bring your innovative gastroenterology device to the Indian market seamlessly and compliantly.