CDSCO License for General-purpose manually-operated operation table
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A completely mobile surgical table (general-purpose) that has been improved to make it usable for almost all parts of the body that require surgery. Manual or hydraulic operation.
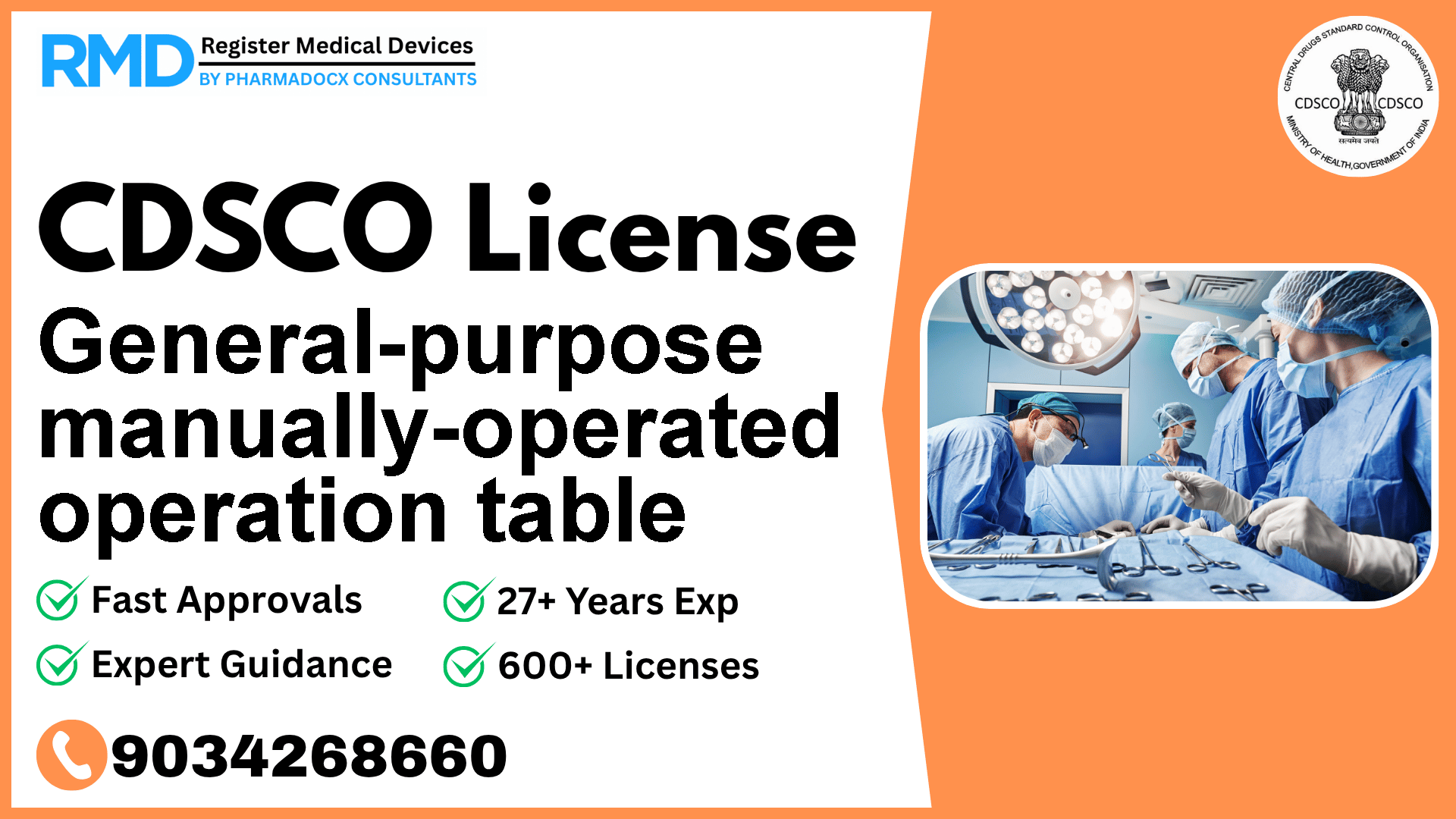
Introduction: Understanding Your General-Purpose Manually-Operated Operation Table and Regulatory Importance
The general-purpose manually-operated operation table is an essential device in the operation theatre, designed for versatile use across various surgical procedures. Featuring manual or hydraulic operation, this mobile surgical table enhances the surgical environment by providing ease of positioning and mobility. Given its application directly in surgical settings, regulatory compliance is critical to ensure patient safety and meet Indian medical device standards.
With the CDSCO notification (File No. 29/Misc/03/2020-DC (199), dated 13.9.2021), this device is classified under Risk Class A, the lowest risk category. Even so, obtaining the appropriate CDSCO license is mandatory for manufacturing or importing to the Indian market. We bring over 25 years of expertise, having assisted more than 500 manufacturers and importers in navigating the CDSCO licensing landscape seamlessly.
CDSCO Regulatory Framework for General-Purpose Manually-Operated Operation Tables
In India, the Central Drugs Standard Control Organization (CDSCO) governs the regulation of medical devices under the Medical Device Rules, 2017. Devices are grouped into four risk classes (A to D), with Class A being low risk. The general-purpose manually-operated operation table falls under Class A, requiring a state-level manufacturing license (MD5) and an import license (MD15) if applicable.
Regulatory oversight involves ensuring compliance with quality management systems, safety standards, testing protocols, and proper documentation before granting licenses. The CDSCO MD Online Portal streamlines application submissions, audits, and correspondence.
Risk Classification and License Requirements for Your Device
- Risk Class: A (Low risk)
- Applicable License: Manufacturing License – MD5 (Application Form MD3) granted by State Licensing Authority
- Import License: MD15 if importing the device into India, granted by Central Licensing Authority
This classification mandates a relatively straightforward process compared to higher-risk devices but still demands thorough preparation.
Manufacturing License Process (MD5) for Class A Devices
Obtaining the MD5 license involves several crucial steps:
Test License (MD13): Before applying for MD5, manufacturers must obtain a test license on Form MD13, allowing product testing. This phase takes approximately 1.5 to 2 months.
Product Testing: Conduct testing at government-approved laboratories to validate compliance with Indian standards. Refer to the list of testing laboratories for authorized facilities.
Document Preparation: Compile all required documents, including technical and quality management files.
Application Submission: Submit the MD5 application (Form MD3) via the CDSCO MD Online Portal.
Audit by Notified Body: A mandatory audit conducted by a notified body verifies compliance with manufacturing and quality system requirements. You can check the list of notified bodies for audit services.
Resolution of Queries: Address any queries raised during the audit or by the licensing authority.
Grant of License: Upon successful completion, the license is granted on Form MD5.
The entire process typically spans 3 to 4 months.
Manufacturing License Documents Required for Class A Operation Tables
To ensure a smooth application process, prepare the following documents meticulously:
- Company Constitution (Incorporation Certificate, Memorandum & Articles of Association)
- Proof of ownership or lease of manufacturing premises
- Details and qualifications of technical staff
- Fire NOC and Pollution Control Board NOC
- Device Master File detailing design, specifications, and manufacturing process (Device Master File Guide)
- Plant Master File describing the manufacturing facility and quality systems (Plant Master File Guide)
- Essential Principles Checklist affirming compliance with safety and performance requirements
- Risk Management File documenting hazard analysis and risk controls (Risk Management Guide)
- Test Reports from government-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documents, typically ISO 13485:2016 certified
Ensuring these documents are comprehensive and accurate reduces delays.
Import License Process (MD15) for Class A Devices
If you intend to import the operation table into India, the MD15 import license issued by the Central Licensing Authority is mandatory. The process includes:
Document Preparation: Collect all required documents such as manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE Certificate (if applicable), Device and Plant Master Files, wholesale license, and company constitution.
Application Submission: Submit Form MD14 via the CDSCO MD Online Portal.
Queries and Clarifications: Respond promptly to any queries raised by the department.
Grant of License: After verification, the MD15 license is granted.
The import license process generally takes 5 to 6 months.
Import License Documents Required
- Valid Manufacturing License of the device from the country of origin
- Free Sale Certificate or Certificate of Marketability
- ISO 13485:2016 Certificate
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale license for distribution in India
- Company Constitution Documents
Thorough documentation ensures timely approvals.
Timeline and Processing Duration
| Process Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 2 to 3 weeks |
| Document Preparation | 2 to 4 weeks |
| MD5 License Application | 3 to 4 months total |
| MD15 Import License | 5 to 6 months total |
Understanding these timelines helps manufacturers plan market entry strategically.
Government Fees and Costs
MD5 Manufacturing License:
- Application fee: Rs. 5,000 per application
- Product fee: Rs. 500 per product
MD15 Import License:
- For Class A devices: 50 per product (approximate INR equivalent)
Additional costs include testing fees at government-approved laboratories, audit fees charged by notified bodies, and consultancy fees if opting for expert assistance.
Common Challenges and Solutions
Incomplete Documentation: Many applicants face delays due to missing or incorrect documents. Solution: Use detailed checklists and consult experienced regulatory professionals.
Delays in Product Testing: Testing facilities may have backlogs. Solution: Schedule testing early and choose notified labs with shorter turnaround times.
Audit Non-Compliance: Audit failures often stem from inadequate QMS implementation. Solution: Perform internal audits and corrective actions before the notified body audit.
Query Management: Delayed responses to CDSCO queries prolong licensing. Solution: Assign a dedicated team member or consultant to handle regulatory communications promptly.
Expert Consultation and Support
With over 25 years of regulatory consultancy experience, we have helped more than 500 companies successfully obtain CDSCO licenses for devices like the general-purpose manually-operated operation table. Our services include:
- End-to-end application preparation and submission
- Assistance with test license procurement and product testing coordination
- Device and Plant Master File development
- Risk management documentation
- Audit readiness and liaison with notified bodies
- Query resolution and follow-up with CDSCO authorities
Partnering with us reduces complexities and accelerates your time-to-market.
Getting Started with Your CDSCO License Application
Assess Your Device Classification: Confirm your device is Class A as per the CDSCO notification.
Initiate Test License (MD13) Application: Begin by applying for the test license to conduct mandatory product testing.
Identify Government-Approved Testing Labs: Schedule your product testing early to avoid bottlenecks.
Prepare Comprehensive Documentation: Utilize our Device Master File and Plant Master File guides to compile accurate dossiers.
Submit Your Application via the CDSCO Portal: Use the CDSCO MD Online Portal for all submissions to ensure compliance with current procedures.
Schedule and Prepare for the Notified Body Audit: Identify a notified body from the official list and prepare for the audit.
Respond Promptly to Queries: Maintain open communication with the licensing authority to expedite approvals.
Your journey to licensing your general-purpose manually-operated operation table in India can be smooth with proper planning, documentation, and expert guidance. Reach out to us to leverage our proven track record and industry insights.
For more detailed insights, consider exploring our MD5 License Guide, which offers step-by-step instructions tailored for Class A device manufacturers.