CDSCO License for Gynaecological examination/treatment table
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
To support a woman's body in the appropriate positions during gynaecological examinations.
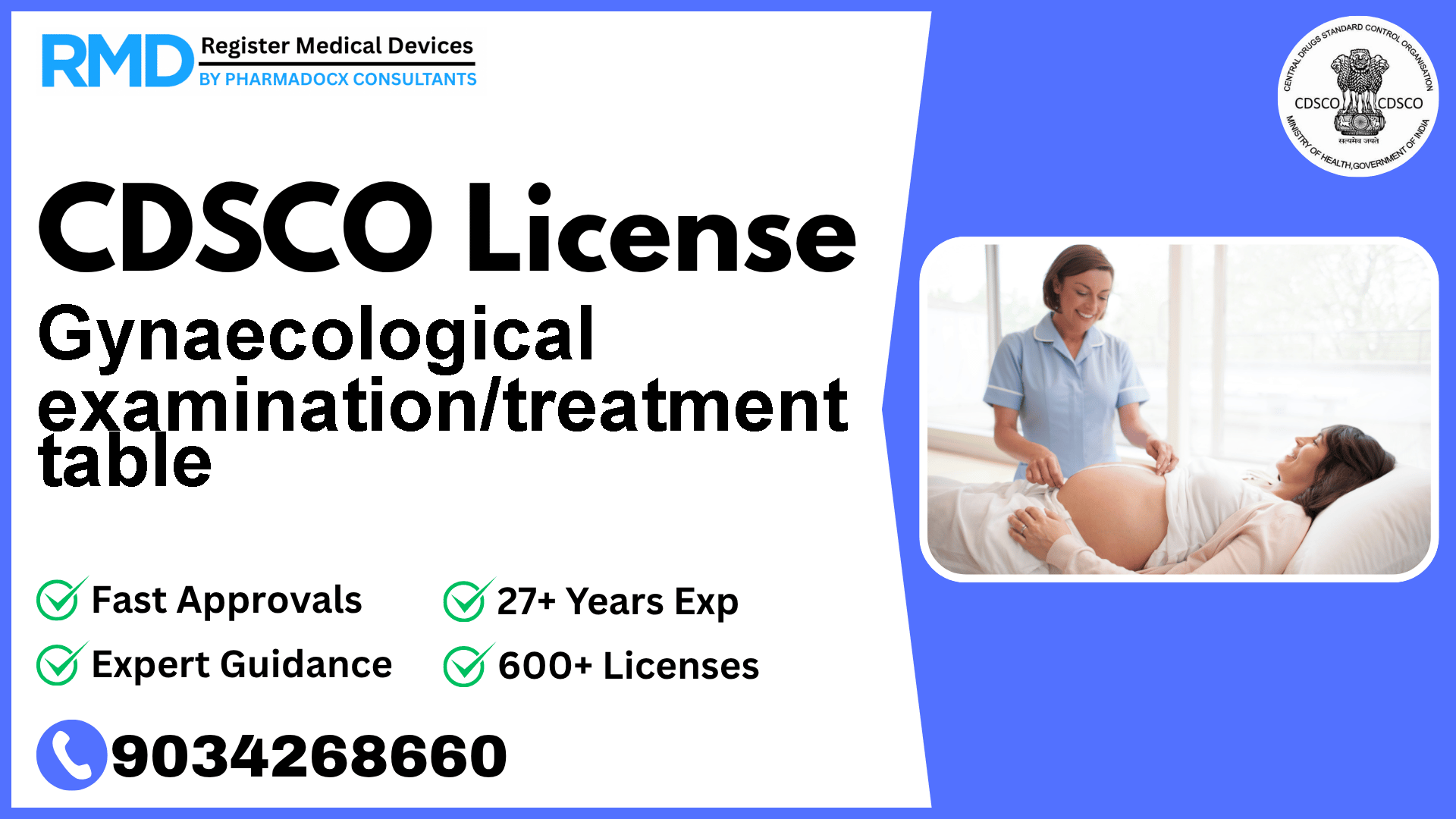
Comprehensive Guide to CDSCO Licensing for Gynaecological Examination/Treatment Tables (Class A Medical Device)
As a trusted regulatory consultancy with over 25 years of experience and having supported 500+ companies in obtaining CDSCO licenses, we understand the complexities manufacturers and importers face in India’s medical device market. This guide focuses on the regulatory journey for the Gynaecological examination/treatment table, a Class A medical device under the Obstetrical and Gynecological category, notified under File No. 29/Misc./03/2020-DC (181) dated 03.6.2022.
Introduction to Gynaecological Examination/Treatment Tables and Their Regulatory Importance
The Gynaecological examination/treatment table is an essential medical device designed to support a woman’s body in various positions during gynecological examinations and treatments. Although classified as a low-risk (Class A) device, obtaining the correct CDSCO license is mandatory for legal manufacturing and marketing in India. The regulatory framework ensures patient safety through quality control, proper manufacturing practices, and compliance with essential principles.
CDSCO Regulatory Framework for Gynaecological Examination/Treatment Tables
The Central Drugs Standard Control Organisation (CDSCO) governs medical device approvals in India. Post the 2020 notification, devices like the Gynaecological examination/treatment table have been classified under Class A, necessitating an MD5 manufacturing license granted by the State Licensing Authority.
Manufacturers must comply with the Medical Device Rules, 2017 (amended), which outline licensing, testing, labeling, and Quality Management System (QMS) requirements.
Risk Classification and License Requirements for Class A Devices
Medical devices in India are categorized into four classes (A to D) based on risk. Class A devices pose the lowest risk and include non-invasive devices like examination tables. For Class A devices such as the Gynaecological examination/treatment table, an MD5 license is required for manufacturing.
- License Type: MD5
- Application Form: MD3
- Licensing Authority: State Licensing Authority
- Total Processing Time: Approximately 3-4 months
- Government Fees: Rs 5,000 per application + Rs 500 per product
If you wish to manufacture multiple models or variants, each product requires separate fees.
Manufacturing License Process (MD5) for Gynaecological Examination/Treatment Tables
The MD5 license process involves several stages:
Test License (Form MD13): Before applying for the MD5 license, you must obtain a test license. This allows sample testing of your device in government-approved labs and takes around 1.5 to 2 months.
Product Testing: The device samples are sent for mandatory testing in government-recognized laboratories to validate compliance with applicable standards. You can refer to the Testing Laboratories list for approved labs.
Documentation Preparation: Prepare a comprehensive set of documents including Device Master File, Plant Master File, Risk Management File, Essential Principles Checklist, and QMS documents.
Submission of MD5 Application (Form MD3): After successful testing, submit the MD5 license application through the CDSCO MD Online Portal.
Audit by Notified Body: A mandatory audit of your manufacturing facility is conducted by a notified body to verify compliance. Check the list of notified bodies.
Query Resolution: Address any queries raised by the department or notified body promptly.
License Grant: Upon successful audit and query resolution, the MD5 license is granted.
Manufacturing License Documents Required for Gynaecological Tables
For your Class A device, the documentation package should include:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed design, specifications, and manufacturing process documentation. Our Device Master File guide can help you prepare this.
- Plant Master File (PMF): Information about manufacturing facilities and quality systems. Learn more with our Plant Master File guide.
- Essential Principles Checklist (Compliance with Indian Medical Device Rules)
- Risk Management File (as per ISO 14971 principles)
- Test Reports from Government-Approved Labs
- Labels and Instructions for Use (IFU)
- Quality Management System Documents (e.g., ISO 13485:2016 certification)
Import License Process (MD15) for Gynaecological Examination/Treatment Tables
If you are an importer rather than a manufacturer, you will need an MD15 import license issued by the Central Licensing Authority. The process typically takes 5-6 months and requires submission of:
- Manufacturing License of the foreign manufacturer
- Free Sale Certificate
- ISO 13485:2016 and CE Certificates
- Device Master File and Plant Master File
- Wholesale license in India
- Company Constitution
The application is submitted using Form MD14 through the CDSCO MD Online Portal.
Government fees vary by device class, with Class A import fees approximately 50 per product.
More detailed guidance on import licensing is available in our Import License Guide.
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Document Preparation | 2 - 3 weeks |
| MD5 Application Processing | 1 - 1.5 months |
| Audit and Query Resolution | 3 - 4 weeks |
| Total Duration | Approx. 3 to 4 months |
Government Fees and Costs
For the Gynaecological examination/treatment table (Class A device):
- Application fee: Rs. 5,000
- Per product fee: Rs. 500
- Additional costs:
- Testing fees charged by government labs (variable)
- Notified body audit fees (varies by body)
- Consultancy and document preparation fees (if outsourced)
Common Challenges and Practical Solutions
Challenge: Delays due to incomplete documentation or improper test reports.
Solution: Engage expert consultants early to prepare all required documents meticulously. Refer to our detailed Risk Management guide to ensure compliance.
Challenge: Difficulty in scheduling audits with notified bodies.
Solution: Identify and communicate with notified bodies listed on the CDSCO portal well in advance.
Challenge: Non-conformities raised during audits.
Solution: Maintain a robust Quality Management System and conduct internal audits before the official audit.
Expert Consultation and Support
Navigating the CDSCO licensing landscape can be daunting. Our team has successfully guided over 500 companies through the MD5 licensing process for Class A devices like the Gynaecological examination/treatment table.
We offer:
- Comprehensive gap analysis
- Documentation and dossier preparation
- Coordination with notified bodies and testing labs
- Post-licensing compliance support
Getting Started with Your CDSCO License Application
Register on the CDSCO MD Online Portal: Start your application process by registering your company on the CDSCO MD Online Portal.
Apply for Test License (Form MD13): Prepare your product samples and initiate the test license application.
Identify Testing Laboratories: Choose government-approved testing labs from the official Testing Laboratories list to conduct product testing.
Prepare Documentation: Begin compiling your Device Master File, Plant Master File, risk assessments, and QMS documentation.
Plan for Notified Body Audit: Schedule an audit with a notified body from the list of notified bodies.
Submit MD5 Application: After successful testing and audit preparations, submit your MD5 license application (Form MD3) through the portal.
By following these actionable steps and leveraging expert guidance, you can efficiently navigate the CDSCO licensing process and bring your Gynaecological examination/treatment table to the Indian market.
For personalized assistance and end-to-end support, please contact our regulatory experts.