CDSCO License for Haidinger brush imager
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
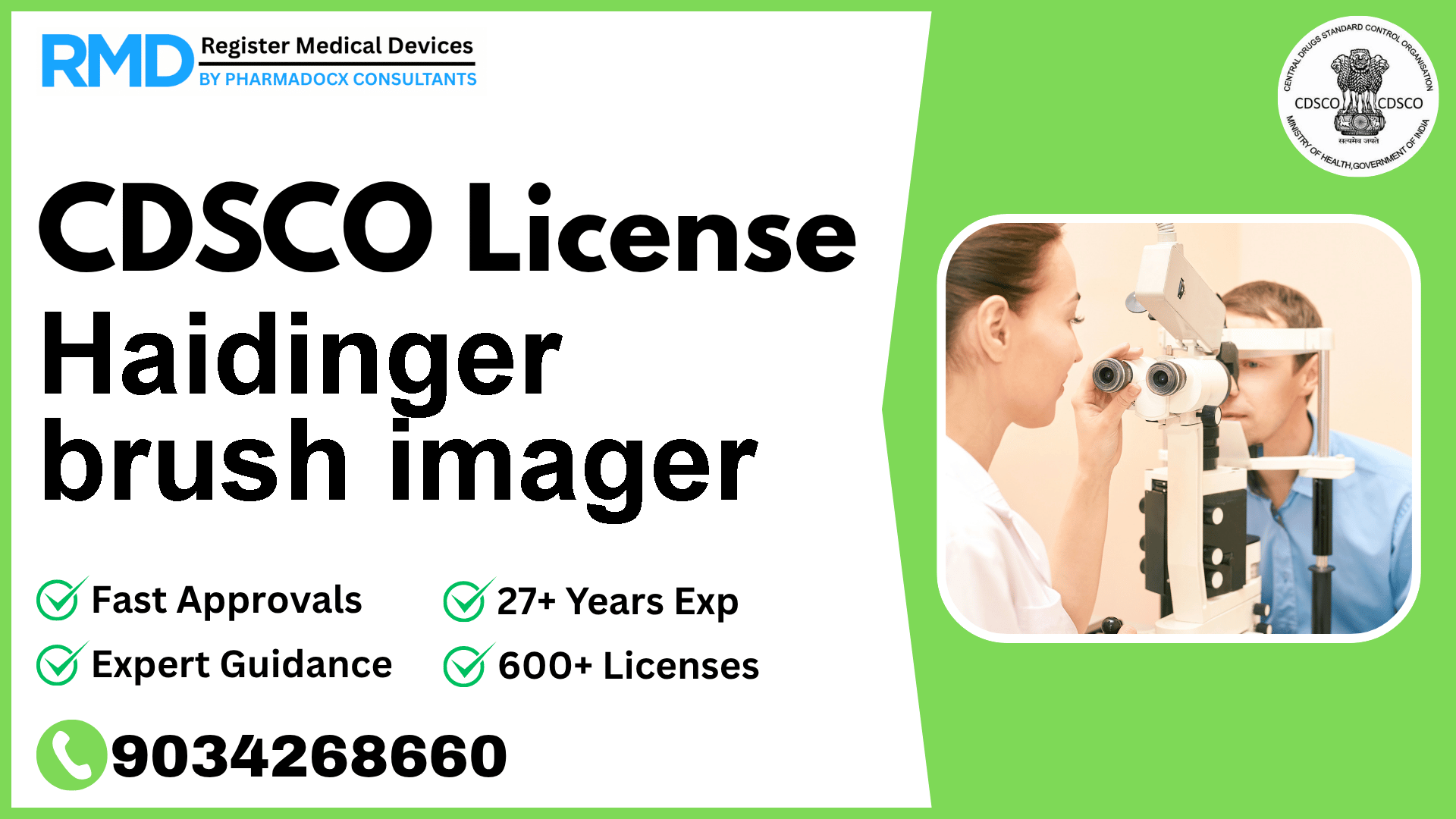
An ophthalmic device designed to produce an image which facilitates his/her visual function evaluation, particularly the macular integrity.
Comprehensive Guide to CDSCO Licensing for Haidinger Brush Imager (Class A Ophthalmic Device)
Navigating the regulatory landscape of medical devices in India can be complex, especially for specialized ophthalmic instruments like the Haidinger brush imager. With over 25 years of experience guiding 500+ companies to successful CDSCO license approvals, we provide you a step-by-step, expert roadmap tailored for this Class A device. This guide covers everything from regulatory frameworks and risk classification to timelines, fees, and common hurdles — empowering manufacturers and importers to confidently enter the Indian market.
Understanding the Haidinger Brush Imager and Its Regulatory Significance
The Haidinger brush imager is an ophthalmic medical device designed to produce images that facilitate the evaluation of visual function, particularly assessing the macular integrity. Given its diagnostic role in eye care, it falls under CDSCO’s Class A risk category, meaning it is considered low risk but still requires compliance with mandatory licensing and quality standards.
India’s regulatory authority, the Central Drugs Standard Control Organization (CDSCO), ensures that devices like the Haidinger brush imager meet safety, efficacy, and quality standards before entering the market. Compliance with CDSCO not only guarantees legal market access but also builds trust with healthcare providers and patients.
CDSCO Regulatory Framework for Ophthalmic Devices like Haidinger Brush Imager
Under the Medical Device Rules (MDR) notified by CDSCO, ophthalmic devices are regulated based on risk classification. The Haidinger brush imager, notified under Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021, is categorized as Class A, the lowest risk class, which simplifies certain procedural aspects but still mandates a manufacturing license.
The licensing process for Class A devices is governed by the State Licensing Authority, distinguishing it from higher-risk categories regulated centrally.
Risk Classification and License Requirements for Haidinger Brush Imager
- Risk Class: A (Low risk)
- License Type: MD5 Manufacturing License (Form MD3)
- Licensing Authority: State Licensing Authority
- Total Timeline: Approximately 3 to 4 months
For Class A devices, obtaining an MD5 license involves several key steps including test license acquisition, product testing, documentation, notified body audit, and final license grant.
Step-by-Step Manufacturing License Process (MD5) for Haidinger Brush Imager
- Apply for Test License (Form MD13):
- Duration: 1.5 to 2 months
- Purpose: Allows you to conduct initial testing and evaluation of the Haidinger brush imager
- Product Testing:
- Conduct tests at government-approved laboratories to validate safety and performance.
- Reference the list of CDSCO-approved testing labs.
- Document Preparation:
- Compile technical dossiers, including Device Master File (DMF), Plant Master File (PMF), risk management reports, and quality management system (QMS) documents.
- Apply for Manufacturing License (Form MD3):
- Submit the application through the CDSCO MD Online Portal.
- Audit by Notified Body:
- A notified body inspects your manufacturing premises and QMS.
- Consult the list of notified bodies for authorized auditors.
- Resolve Queries:
- Address any observations or requests for additional information raised by the licensing authority or notified body.
- License Grant (Form MD5):
- Upon satisfactory compliance, the state authority issues the MD5 manufacturing license.
Manufacturing License Documents Required for Haidinger Brush Imager
To ensure a smooth application process, prepare the following documents meticulously:
- Company Constitution or Incorporation Certificate
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualification of Technical Staff
- Fire Safety NOC
- Pollution Control Board NOC
- Device Master File (DMF) – See our detailed Device Master File Guide
- Plant Master File (PMF) – Guidance available here
- Essential Principles Checklist confirming compliance with MDR
- Risk Management File documenting hazard identification and mitigation strategies (Risk Management Guide)
- Product Test Reports from CDSCO-approved labs
- Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, typically ISO 13485:2016 certification
Import License Process (MD15) for Haidinger Brush Imager
If you are an importer rather than a manufacturer, you will require an MD15 license granted by the Central Licensing Authority.
- Timeline: Approximately 5 to 6 months
- Application Form: MD14 for MD15 license
- Documents Required:
- Valid Manufacturing License from country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate
- Device Master File and Plant Master File
- Wholesale License
- Company Constitution
- Fees: Class A devices attract a site fee of approx 50 per product
Detailed guidance is available in our Import License Guide.
Timeline and Processing Duration for Haidinger Brush Imager Licensing
| Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | 2 – 3 weeks |
| Application Processing | 1 month |
| Audit and Inspection | 3 – 4 weeks |
| Query Resolution | 2 – 3 weeks |
| Total | 3 to 4 months |
This timeline assumes prompt response to queries and no major deficiencies during audit.
Government Fees and Costs Breakdown
- MD5 License Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product (for each variant of Haidinger brush imager)
- Testing Charges: Variable depending on lab, typically Rs 30,000 to Rs 50,000 per device
- Notified Body Audit Fee: Depends on the body; typically ranges from Rs 50,000 to Rs 1,00,000
Budgeting realistically for Rs 1.5 to 2 lakhs ensures you cover all mandatory expenses.
Common Challenges and Practical Solutions
- Incomplete Documentation: Many applicants underestimate the importance of detailed DMFs and PMFs. Utilize professional templates and consult our Plant Master File Guide to avoid rejections.
- Delayed Testing Results: Pre-book testing slots at CDSCO-approved labs to prevent bottlenecks.
- Non-Compliance During Audit: Engage a notified body early to conduct pre-audit assessments.
- Query Resolution Delays: Assign dedicated regulatory personnel to respond promptly to departmental queries.
Expert Consultation and Support
Given the technical intricacies and evolving regulations, expert consultancy can substantially reduce approval time and cost overruns. Our team has successfully navigated the CDSCO licensing process for over 500 medical device companies, including ophthalmic devices like the Haidinger brush imager.
We offer:
- Comprehensive dossier preparation
- Liaison with notified bodies and CDSCO officials
- Pre-audit readiness assessments
- Regulatory strategy for product variants
Getting Started with Your CDSCO License Application for Haidinger Brush Imager
- Assess Your Device Classification: Confirm your device as Class A using the Medical Device Classification tool.
- Prepare Test License Application: Gather preliminary documents and submit Form MD13 on the CDSCO MD Online Portal.
- Schedule Product Testing: Contact CDSCO-approved labs early to secure testing dates.
- Compile Technical Documentation: Develop Device and Plant Master Files alongside risk management and QMS documents.
- Engage a Notified Body: Choose a notified body from the official list and schedule your audit.
- Submit Manufacturing License Application: File Form MD3 through the CDSCO portal.
- Respond Promptly to Queries: Maintain open communication channels for efficient resolution.
By following these actionable steps and leveraging expert support, you can streamline the licensing of your Haidinger brush imager and access India's vast ophthalmic device market with confidence.
For personalized assistance and turnkey licensing solutions, connect with our regulatory experts today.