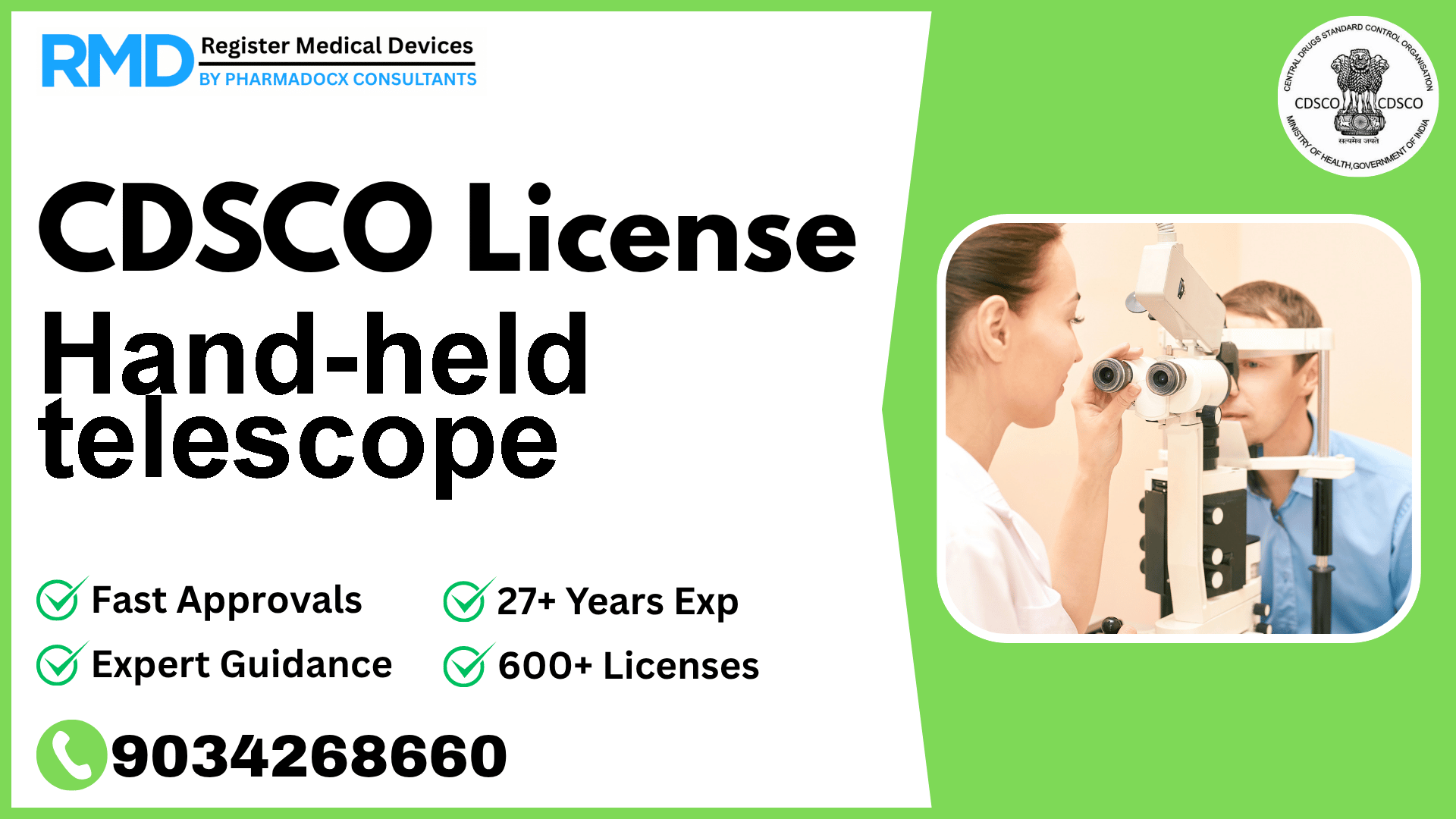
CDSCO License for Hand-held telescope
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A device that consists of an arrangement of ophthalmic lenses or mirrors with a handle intended to enlarge images for a visually impaired patient/person.
Comprehensive Guide for CDSCO Licensing of Hand-held Telescope (Class A Medical Device in Ophthalmology)
As seasoned regulatory consultants with over 25 years of experience and having assisted 500+ companies in securing CDSCO licenses, we understand the intricacies involved in navigating the Indian medical device regulatory landscape. The hand-held telescope, an ophthalmic device designed to aid visually impaired patients by enlarging images, is classified as a Class A device under the CDSCO framework. This classification impacts the licensing route, timelines, and documentation requirements.
Understanding the Hand-held Telescope and Its Regulatory Significance
The hand-held telescope is a vital ophthalmic tool that enhances the quality of life for visually impaired individuals by magnifying images. Given its direct application in patient care, regulatory oversight ensures the device meets safety and performance standards. The Government of India formally notified this device under Fts No. 29/MiscJO3/2020-DC (187) on 9.8.2021, mandating compliance with CDSCO regulations.
CDSCO Regulatory Framework for Hand-held Telescope (Class A Device)
Class A devices are considered low risk, and the CDSCO mandates the issuance of an MD5 manufacturing license granted by the State Licensing Authority. The entire process includes obtaining a test license (MD13), product testing, audit by a notified body, and final license approval. Importers require an MD15 import license, which involves a separate process outlined later.
To start your application process, manufacturers should use the CDSCO MD Online Portal for all submissions.
Risk Classification and License Requirements
- Device: Hand-held telescope
- Risk Class: A (Low risk)
- License Type: MD5 Manufacturing License (Form MD3)
- Regulatory Authority: State Licensing Authority
For detailed classification criteria, consult the Medical Device Classification guide.
Manufacturing License Process for Hand-held Telescope (MD5 License)
The MD5 license process for Class A devices generally takes about 3 to 4 months and involves the following steps:
Obtain Test License (MD13): Submit Form MD13 application to the CDSCO to get permission for product testing. This takes approximately 1.5 to 2 months.
Product Testing: Conduct product testing at government-approved labs. Testing ensures compliance with essential principles.
Document Preparation: Prepare and compile all required documentation, including Device Master File and Plant Master File.
Submit MD5 Application: File the manufacturing license application using Form MD3 through the online portal.
Audit by Notified Body: Arrange an audit with a notified body to verify compliance.
Query Resolution: Address any queries raised by the licensing authority or the notified body promptly.
License Grant: Upon satisfactory audit and document review, the MD5 license is issued.
Manufacturing License Documents Required for Hand-held Telescope
- Company Constitution Documents (e.g., MOA, AOA)
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Qualification and Experience Documents of Technical Staff
- Fire NOC and Pollution Control NOC
- Device Master File (DMF) detailing device specifications and design. Our Device Master File guide can assist with preparation.
- Plant Master File (PMF) describing manufacturing processes. Refer to our Plant Master File guide.
- Essential Principles Checklist demonstrating compliance with CDSCO standards
- Risk Management File highlighting identified risks and mitigation strategies. We recommend reviewing best practices in Risk Management.
- Test Reports from government-approved laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) Documents, preferably ISO 13485 compliant
Import License Process for Hand-held Telescope (MD15 License)
For importers, the MD15 license issued by the Central Licensing Authority is mandatory. The import license process generally takes 5 to 6 months and includes:
- Document preparation including Manufacturing License, Free Sale Certificate, CE Certificate, ISO 13485:2016 certification, Device and Plant Master Files
- Submission of Form MD14 for license application via the CDSCO MD Online Portal
- Response to any queries raised by CDSCO
- Final license grant
The licensing fees vary based on device class and quantity. More details can be found in the Import License Guide.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate
- Device Master File
- Plant Master File
- Wholesale License
- Company Constitution Documents
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 2 - 3 weeks (approx.) |
| Document Preparation | 2 - 3 weeks |
| MD5 Application Processing | 2 - 3 months |
| Total Time | 3 - 4 months |
Being proactive in document preparation and scheduling audits can significantly reduce delays.
Government Fees and Costs
- MD5 License Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product
Additional costs include testing fees at government-approved labs and notified body audit charges.
Common Challenges and Solutions
- Delayed Testing Results: Plan testing early and choose government-approved labs from the official Testing Laboratories list.
- Incomplete Documentation: Use comprehensive checklists and expert guidance. Our detailed guides on DMF and PMF can streamline preparation.
- Audit Non-compliance: Engage with a notified body listed here with proven expertise in ophthalmic devices.
- Query Resolution Delays: Respond quickly and thoroughly to regulatory queries, supported by expert consultants.
Expert Consultation and Support
Navigating the CDSCO licensing process demands deep regulatory knowledge and experience. Our team has successfully guided over 500 manufacturers and importers through the entire lifecycle—from documentation to audit readiness and query resolution. We provide tailored support for your hand-held telescope device, ensuring regulatory compliance and market readiness.
Getting Started with Your CDSCO License Application
- Pre-assessment: Conduct a gap analysis of your current documentation and QMS against CDSCO requirements.
- Register on CDSCO MD Online Portal: Create an account and familiarize yourself with the submission process.
- Prepare Test License Application (MD13): Gather all required documents and submit early to avoid delays.
- Select Accredited Testing Lab: Choose from the official Testing Laboratories for timely test reports.
- Compile Device and Plant Master Files: Leverage our expert guides to ensure completeness and accuracy.
- Schedule Audit with Notified Body: Engage a notified body well in advance to fit your project timeline.
- Submit MD5 Application: Once test reports and audit readiness are confirmed, apply for the manufacturing license.
Starting early and partnering with experienced consultants can transform a complex process into a smooth pathway to market entry. Contact us to begin your CDSCO licensing journey for your hand-held telescope and ensure compliance with all regulatory mandates efficiently.