CDSCO License for Indirect binocular ophthalmoscope
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An ophthalmic instrument designed to examine the interior of the eye allowing the examiner to clearly see a wide angle, stereoscopic impression of the details of the fundus (retina) and other structures.
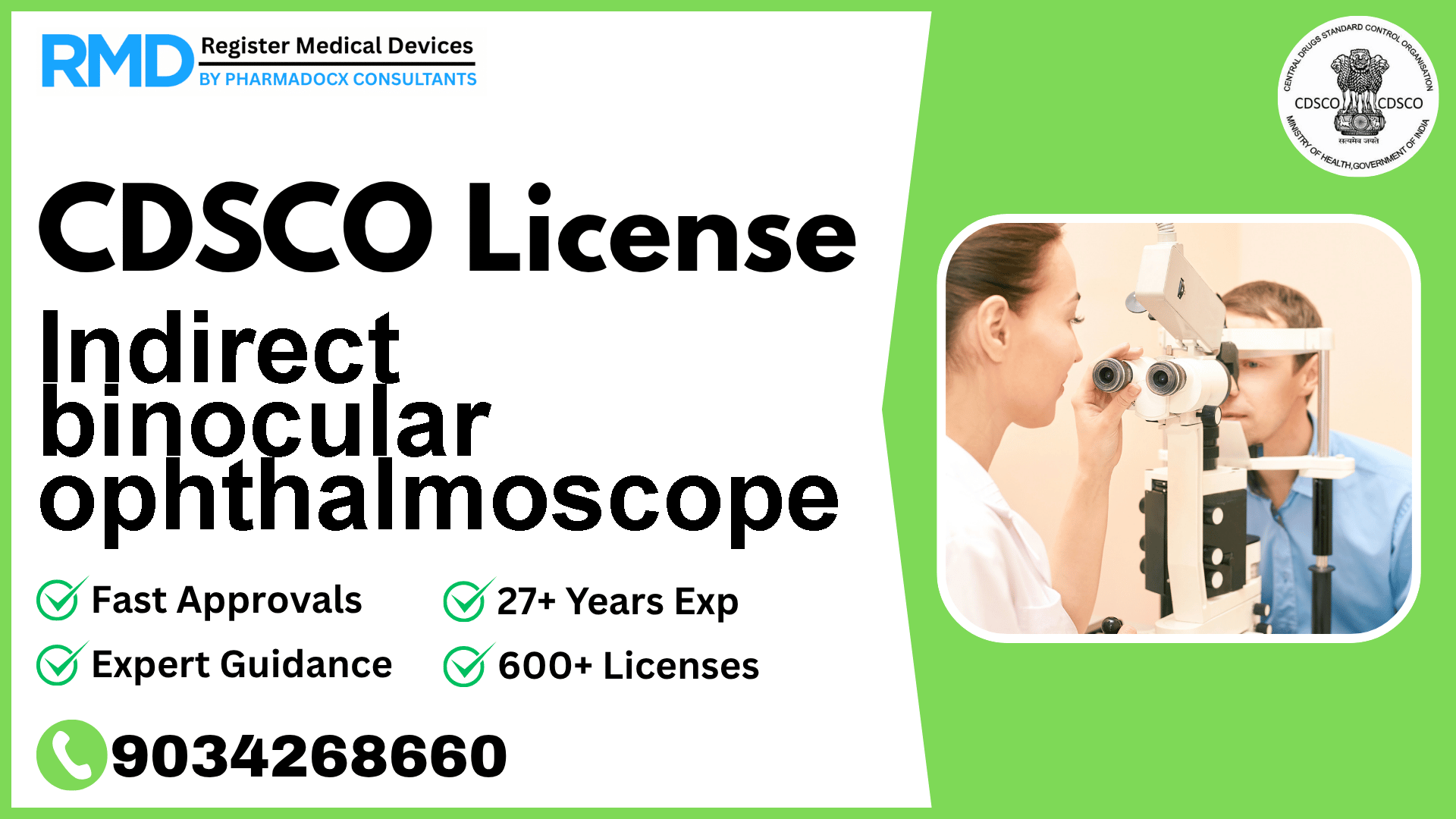
Introduction to Indirect Binocular Ophthalmoscope and Regulatory Importance
An Indirect Binocular Ophthalmoscope is a vital ophthalmic instrument that enables detailed examination of the interior eye structures, including the retina and fundus, delivering a wide-angle stereoscopic view. Given its critical role in eye diagnostics, regulatory compliance with the Central Drugs Standard Control Organization (CDSCO) is mandatory before marketing this device in India. Proper licensing ensures patient safety, product quality, and legal market access.
With over 25 years of experience, we have assisted more than 500 companies in successfully navigating the CDSCO licensing framework for devices like the Indirect Binocular Ophthalmoscope, making this guide a practical resource for manufacturers and importers.
CDSCO Regulatory Framework for Indirect Binocular Ophthalmoscopes
The Indirect Binocular Ophthalmoscope falls under the ophthalmology category and is notified under Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021. As a Class A medical device, it is considered low risk and governed by the State Licensing Authority under the CDSCO regulations. Compliance with Indian Medical Device Rules (MDR) 2017 is mandatory, including obtaining the MD5 manufacturing license.
For detailed classification, refer to our Medical Device Classification guide.
Risk Classification and License Requirements for Class A Devices
Class A devices like the Indirect Binocular Ophthalmoscope are categorized as low risk. The regulatory pathway requires:
- Manufacturing License (MD5): Granted by the State Licensing Authority.
- Test License (MD13): Initial license to allow product testing.
- Product Testing: Conducted at government-approved laboratories.
The entire process typically spans 3-4 months.
Manufacturing License Process (MD5) for Indirect Binocular Ophthalmoscopes
Obtain Test License (Form MD13): Apply via the CDSCO MD Online Portal. The test license allows you to legally test your device in certified labs. This stage takes approximately 1.5 to 2 months.
Product Testing: Submit your device samples to government-approved testing laboratories. You can find the list of authorized labs on the CDSCO website Testing Laboratories.
Document Preparation: Compile all required documents including Device Master File, Plant Master File, and Risk Management File.
Apply for Manufacturing License (Form MD3): Submit your application for the MD5 license through the CDSCO MD Online Portal.
Audit by Notified Body: An audit is conducted to verify your quality management system and manufacturing premises. Refer to the Notified Bodies List for authorized auditors.
Resolution of Queries: Address any observations or queries raised by the licensing authority or notified body.
Grant of MD5 License: Upon successful compliance, you will receive the manufacturing license on Form MD5, allowing you to manufacture and market the device in India.
Manufacturing License Documents Required for Class A Devices
Preparing accurate and comprehensive documentation is key. For the Indirect Binocular Ophthalmoscope, you will need:
- Company Constitution Documents (e.g., Incorporation Certificate)
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire Safety NOC
- Pollution Control NOC
- Device Master File (DMF): Detailed product specifications and design data. Our Device Master File guide can assist you.
- Plant Master File (PMF): Manufacturing processes and quality controls. See our Plant Master File Guide for best practices.
- Essential Principles Checklist confirming compliance with Indian MDR
- Risk Management File demonstrating hazard analysis and mitigation. Learn more about Risk Management
- Test Reports from government-approved labs
- Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documents, typically ISO 13485:2016 certified
Import License Process (MD15) for Indirect Binocular Ophthalmoscopes
If you intend to import the device instead of manufacturing locally, the MD15 license issued by the Central Licensing Authority is required. The process involves:
- Document preparation including Manufacturing License, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device Master File, Plant Master File, Wholesale License, and Company Constitution.
- Application via the CDSCO MD Online Portal.
- Query resolution.
- License issuance within 5-6 months.
The MD15 import license fees for Class A devices are approximately 50 per product.
For comprehensive details, see our Import License Guide.
Timeline and Processing Duration
- Test License (MD13): 1.5 - 2 months
- Product Testing: 3-4 weeks depending on lab capacity
- Document Preparation: Concurrent with testing, 3-4 weeks
- MD5 License Application and Audit: 4-6 weeks
- Total Estimated Duration: Approximately 3 to 4 months for complete MD5 license issuance
Planning these stages efficiently helps minimize delays.
Government Fees and Costs
For Class A devices such as the Indirect Binocular Ophthalmoscope:
- Test License (MD13): Included in application process
- Manufacturing License (MD5): Rs 5,000 per application
- Product Fee: Rs 500 per product
Additional costs include notified body audit fees and testing laboratory charges, which vary by provider.
Common Challenges and Solutions
- Incomplete Documentation: Ensure all files such as Device Master File and Risk Management File are thorough and compliant. Using expert templates can prevent rejections.
- Delays in Product Testing: Book slots early with government-approved labs and prepare samples meticulously.
- Audit Non-Compliance: Conduct internal audits prior to notified body visits to identify gaps.
- Query Resolution Delays: Respond promptly with detailed explanations and supporting evidence.
We recommend engaging consultants with extensive CDSCO experience to navigate these challenges smoothly.
Expert Consultation and Support
With over 25 years of regulatory expertise and a track record of supporting 500+ manufacturers and importers, we offer end-to-end assistance including:
- Licensing strategy and classification
- Document preparation and review
- Coordination with notified bodies and testing labs
- Audit preparation and training
- Query management and follow-ups
Our clients benefit from accelerated approvals and reduced compliance risks.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm Class A status using official CDSCO guidelines.
- Prepare Preliminary Documentation: Start compiling your Device Master File, Plant Master File, and Risk Management File.
- Apply for Test License (MD13): Submit your application through the CDSCO MD Online Portal.
- Engage a Notified Body and Testing Labs Early: Coordinate audit scheduling and sample testing to avoid bottlenecks.
- Leverage Expert Support: Consider partnering with regulatory consultants specializing in ophthalmic devices to ensure compliance and streamline the process.
Embarking on your CDSCO licensing journey for the Indirect Binocular Ophthalmoscope with these concrete steps will position your product for timely market entry and sustained success in India’s regulated medical device landscape.