CDSCO License for Intravenous Catheter
Medical Device Information
Intended Use
A catheter that is inserted into a vein fo r supplying medications or nutrients dir ectly into the bloodstream or for diagno sticpurposes such as studying blood pre ssure
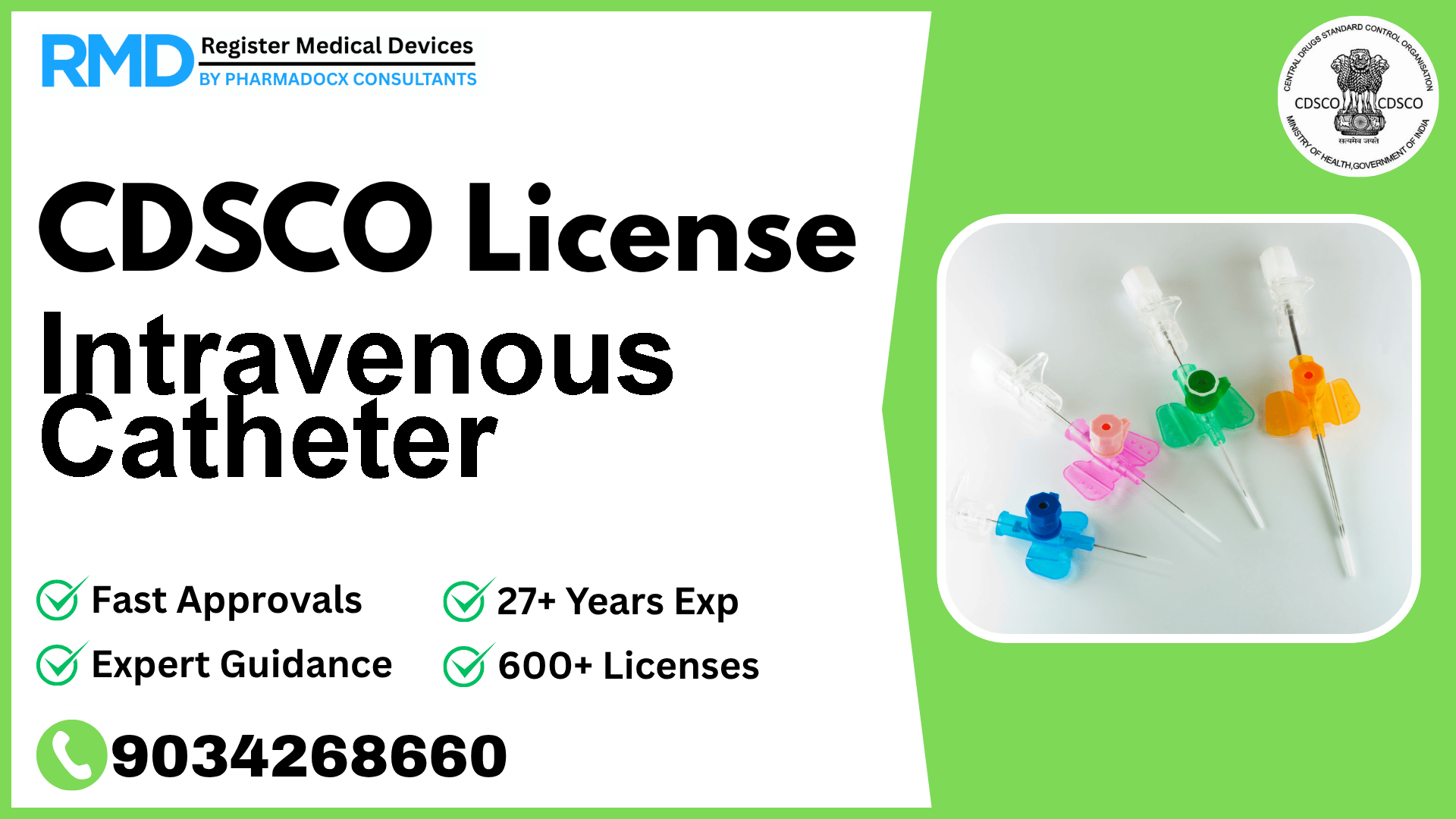
Comprehensive Guide to CDSCO Licensing for Intravenous Catheters (Class B Medical Device)
Intravenous catheters are critical medical devices designed to deliver medications, nutrients, or for diagnostic purposes directly into a patient's bloodstream. Given their invasive nature and direct contact with the vascular system, regulatory oversight by the Central Drugs Standard Control Organization (CDSCO) in India ensures these devices meet stringent quality and safety standards before reaching the market.
With over 25 years of experience assisting 500+ companies navigate the CDSCO licensing landscape, we provide you with an in-depth, practical roadmap to secure your license for Intravenous Catheters, classified as Class B medical devices under the Indian regulatory framework.
CDSCO Regulatory Framework for Intravenous Catheters
Intravenous catheters fall under the category of 'Catheters' notified by CDSCO notification 29/Misc/3/2017-DC (292) dated 06.06.2018. As a Class B device (low-moderate risk), the manufacturing and import of these catheters require adherence to specific guidelines and licensing processes governed by the Medical Device Rules 2017.
The CDSCO framework aims to ensure devices are manufactured under controlled conditions, undergo requisite testing, and comply with quality management systems before being introduced in the Indian market.
Risk Classification and License Requirements for Intravenous Catheters
According to the CDSCO classification, Intravenous Catheters fall under Class B medical devices. This classification demands the following licensing requirements:
- Manufacturing License: MD5 License granted by the State Licensing Authority
- Application Form: MD3
- Test License Requirement: Mandatory MD13 test license prior to manufacturing license
- Risk Management Compliance: Implementation of risk management file as per ISO 14971
Manufacturers must follow the detailed procedure involving product testing, quality audits, and documentation submission to obtain the MD5 license.
Manufacturing License Process (MD5) for Intravenous Catheters
The MD5 license process for Class B devices like intravenous catheters is comprehensive but streamlined for timely approvals. The process involves the following stages:
Apply for Test License (Form MD13): The first step is to obtain a test license allowing you to manufacture devices for testing purposes. This typically takes 1.5 to 2 months.
Product Testing at Government-Approved Laboratories: Get your catheters tested for compliance with Indian and international standards. CDSCO maintains a list of approved testing laboratories for this purpose.
Documentation Preparation: Compile all technical files, including Device Master File (DMF), Plant Master File (PMF), Essential Principles Checklist, Risk Management File, and Quality Management System (QMS) documents.
Apply for Manufacturing License (Form MD3): Submit the application through the CDSCO MD Online Portal.
Audit by Notified Body: The State Licensing Authority will appoint a notified body to audit your manufacturing facilities. You can check the list of notified bodies authorized for MD5 audits.
Resolution of Queries: Address any queries raised by the department or notified body promptly to avoid delays.
Grant of License (Form MD5): Upon successful audit and document verification, the manufacturing license is granted.
Manufacturing License Documents Required for Intravenous Catheters
To ensure a smooth licensing process, prepare the following documents meticulously:
- Company Constitution Documents: Memorandum of Association, Articles of Association, or Partnership Deed
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualification and Experience Records
- Fire Safety NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed technical specifications, manufacturing process, and testing protocols (Guide to Device Master File)
- Plant Master File (PMF): Details of manufacturing environment, equipment, and quality control processes (Plant Master File Guide)
- Essential Principles Checklist: Compliance checklist per Indian Medical Device Rules
- Risk Management File: Evidence of risk analysis and mitigation strategies (Risk Management Implementation)
- Test Reports: From CDSCO-approved testing laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documents: ISO 13485 certifications, SOPs, and quality manuals
Import License Process (MD15) for Intravenous Catheters
If you are an importer of intravenous catheters into India, the MD15 license is mandatory and is governed by the Central Licensing Authority. The process is as follows:
Document Preparation: Ensure you have all relevant certificates including Manufacturing License, Free Sale Certificate, ISO 13485:2016, CE Certificate, DMF and PMF, and Wholesale License.
Application Submission: File the application on the CDSCO MD Online Portal using Form MD14.
Queries Resolution: Respond efficiently to any department queries.
Grant of MD15 License: Typically takes 5-6 months.
Note: Unlike manufacturing licenses, no test license (MD13) is required for import licenses.
Import License Documents Required
- Valid Manufacturing License of the foreign manufacturer
- Free Sale Certificate issued by the country of origin
- ISO 13485:2016 and CE Certificates
- Device Master File and Plant Master File
- Wholesale Drug License
- Company Constitution and Address Proof
Timeline and Processing Duration
| Stage | Duration |
|---|---|
| Test License (Form MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Manufacturing License (MD5) | 1 - 1.5 months |
| Audit and Query Resolution | 0.5 - 1 month |
| Total MD5 Licensing Time | 3 - 4 months approx. |
| Import License (MD15) | 5 - 6 months approx. |
Government Fees and Costs
For Intravenous Catheters (Class B), the fees structure for manufacturing license is as follows:
- Test License (MD13): Rs. 5000
- Manufacturing License Application (MD5): Rs. 5000 per application
- Product Fee: Rs. 500 per product
For import licenses (MD15), fees depend on risk class and number of products:
- Class B: Rs. 2000 per site + Rs. 1000 per product
Budgeting accurately for these fees early in the process avoids surprises.
Common Challenges and Solutions
Challenge 1: Delays in Product Testing
- Solution: Engage with CDSCO-approved testing labs early and submit samples promptly to avoid bottlenecks.
Challenge 2: Incomplete Documentation
- Solution: Use detailed checklists and templates such as our Device Master File guide to ensure completeness.
Challenge 3: Audit Non-Compliance
- Solution: Conduct internal audits before notified body visits and maintain robust QMS documentation.
Challenge 4: Query Resolution Delays
- Solution: Assign a dedicated regulatory liaison to respond quickly and comprehensively to department queries.
Expert Consultation and Support
Navigating CDSCO regulatory pathways can be complex, especially for first-time manufacturers and importers. Our consultancy has guided hundreds of clients through every step—from test licenses to final audits and license issuance—ensuring compliance and minimizing time to market.
We assist with:
- Preparation of customized Device and Plant Master Files
- Risk Management implementation as per ISO 14971
- QMS documentation and ISO 13485 alignment
- Coordination with notified bodies and testing labs
- Timely submission and follow-up on the CDSCO MD Online Portal
Getting Started with Your CDSCO License Application for Intravenous Catheters
Assess your device classification and regulatory obligations using the Medical Device Classification tool.
Prepare and submit your test license application (Form MD13) via the CDSCO MD Online Portal.
Engage with an approved laboratory promptly to initiate product testing.
Compile all necessary documentation, leveraging our comprehensive guides and templates.
Plan and schedule your notified body audit well in advance to avoid delays.
Maintain open communication with CDSCO authorities for swift query resolution.
By following these steps and leveraging expert support, manufacturers and importers can confidently secure their CDSCO licenses for intravenous catheters and establish a strong foothold in the Indian medical device market.
For personalized assistance and turnkey solutions, reach out to our regulatory experts with decades of experience in CDSCO licensing.