CDSCO License for Maddox trial lens
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A special ophthalmic trial lens in the form of a rod or series of rods (grooves/cylinders) that changes the size, shape, and colour of an image to dissociate the eyes in the evaluation of eye muscle dysfunction.
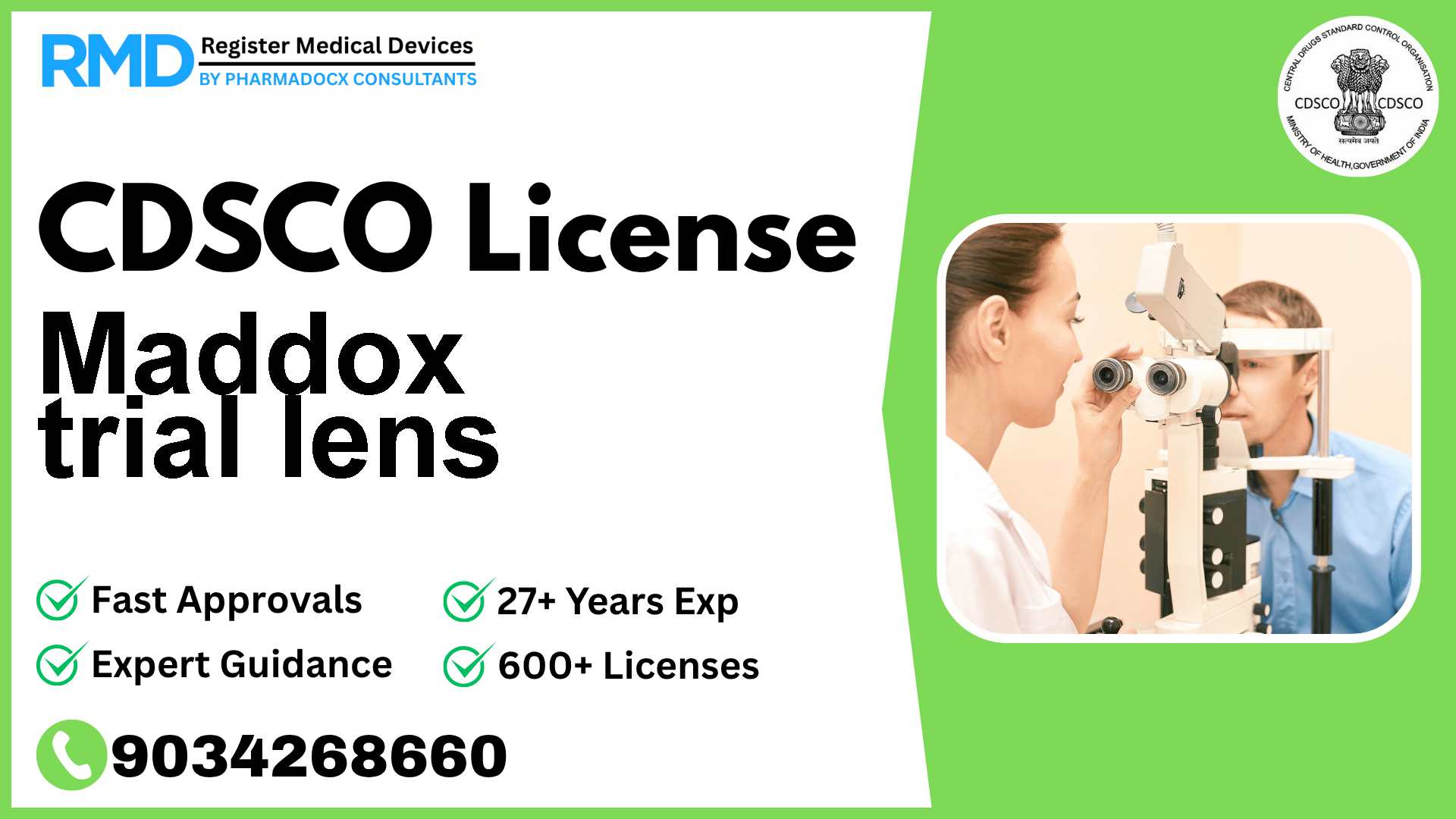
Comprehensive Guide to CDSCO Licensing for Maddox Trial Lens (Class A Medical Device)
Navigating the regulatory landscape for medical devices in India can be complex, especially for specialized ophthalmic devices like the Maddox trial lens. With over 25 years of experience and having supported 500+ companies in securing CDSCO licenses, we provide you with an authoritative, step-by-step guide to obtaining your manufacturing license for this Class A device.
Introduction to Maddox Trial Lens and Regulatory Importance
The Maddox trial lens is a precision ophthalmic instrument designed as a rod or a series of rods with grooves/cylinders, used primarily to dissociate the eyes for evaluating eye muscle dysfunction. It plays a critical role in ophthalmology diagnostics by altering image size, shape, and color to aid in clinical assessments.
Given its diagnostic significance, regulatory compliance is essential to ensure safety, efficacy, and market authorization under the Indian Medical Device Rules (MDR) 2017. The device falls under Class A, the lowest risk category, but still requires a valid CDSCO manufacturing license (MD5) for legal manufacture and sale.
CDSCO Regulatory Framework for Maddox Trial Lens
India’s Central Drugs Standard Control Organization (CDSCO) oversees the regulation of medical devices. The MDR 2017 classifies devices based on risk, with Class A devices regulated primarily by State Licensing Authorities. The regulatory framework mandates registration, licensing, testing, and audits before market entry.
For Maddox trial lenses, the licensing process is streamlined but requires strict adherence to documentation and quality management.
Risk Classification and License Requirements for Maddox Trial Lens
- Device Risk Class: A
- Regulatory Authority: State Licensing Authority
- License Type: MD5 Manufacturing License (Application Form MD3)
- Notification Reference: Fts No. 29/MiscJO3/2020-DC (187), dated 9.8.2021
This classification means the device is considered low risk, but compliance with essential principles and quality standards remains mandatory.
Manufacturing License Process (MD5) for Maddox Trial Lens
The MD5 license process involves multiple steps:
- Test License (Form MD13): Initial permission to manufacture for testing purposes; valid for 1.5 to 2 months.
- Product Testing: Testing must be conducted at CDSCO-approved government laboratories. You can refer to the list of testing laboratories for authorized facilities.
- Documentation Preparation: Compile all required documents including Device Master File, Plant Master File, risk management file, and QMS documents.
- License Application Submission: Submit the application on the CDSCO MD Online Portal using Form MD3.
- Audit by Notified Body: For Class A devices, audits are conducted by notified bodies listed on the Notified Bodies List.
- Query Resolution: Address any queries raised by CDSCO or the notified body promptly.
- Grant of License: Upon satisfactory compliance, the MD5 license is granted on Form MD5.
Manufacturing License Documents Required for Maddox Trial Lens
Preparing a complete and accurate dossier is crucial. Here is the checklist:
- Company Constitution documents (e.g., incorporation certificate)
- Proof of ownership or lease of manufacturing premises
- Qualification and experience certificates of technical staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design and specifications – our detailed Device Master File guide is invaluable here
- Plant Master File (PMF) describing manufacturing processes – learn more in our Plant Master File guide
- Essential Principles Checklist confirming compliance with safety and performance
- Risk Management File aligned with ISO 14971 principles
- Test reports from government-approved laboratories
- Labels and Instructions for Use (IFU) complying with regulatory standards
- Quality Management System (QMS) documents, preferably ISO 13485 certified
Import License Process (MD15) – Not Applicable for Manufacturers
For manufacturers intending to import Maddox trial lenses, an MD15 import license is required from the Central Licensing Authority. This process is more involved and takes about 5-6 months. However, since this guide focuses on manufacturing in India, the MD5 license process is pertinent.
For importers, detailed guidance is available here: Import License Guide.
Timeline and Processing Duration
- Test License (MD13): 1.5 to 2 months
- Product Testing: 3-4 weeks depending on lab capacity
- Audit and Document Review: 4-6 weeks
- Final Grant of MD5 License: Within 3-4 months from initial application
Manufacturers should plan for approximately 3 to 4 months from start to finish, assuming timely response to queries.
Government Fees and Costs
- Application Fee: Rs. 5,000 per application
- Product Fee: Rs. 500 per product
Additional costs may include fees for testing, notified body audit charges, and internal QMS implementation.
Common Challenges and Solutions
Challenge: Delays in test reports due to backlogs at government labs.
Solution: Engage early with testing labs and consider pre-audit readiness checks to avoid surprises.
Challenge: Incomplete documentation causing repeated queries.
Solution: Use checklists and expert reviews before submission. Our MD5 License Guide provides detailed documentation tips.
Challenge: Difficulty in coordinating notified body audits.
Solution: Schedule audits well in advance and maintain clear communication with the notified body.
Expert Consultation and Support
With our vast experience assisting over 500 companies, we understand the nuances of the CDSCO licensing process for ophthalmic devices like the Maddox trial lens. We offer tailored consulting services including dossier preparation, audit readiness, and regulatory strategy to ensure a smooth, expedited approval.
Getting Started with Your CDSCO License Application
- Assess your product classification and confirm it as Class A with reference to the Medical Device Classification.
- Register on the CDSCO MD Online Portal and initiate the test license application (Form MD13).
- Prepare your Device and Plant Master Files using our comprehensive guides.
- Identify and book testing slots at government-approved labs.
- Compile all necessary documentation and QMS files.
- Submit your MD5 manufacturing license application (Form MD3) through the portal.
- Coordinate audit scheduling with a notified body listed here: Notified Bodies List.
- Respond promptly to any CDSCO or auditor queries to avoid delays.
Beginning your regulatory journey well-prepared can significantly reduce time to market and compliance risks. Reach out to our expert team for personalized assistance and ensure your Maddox trial lens is ready to serve the Indian ophthalmology market with full CDSCO compliance.