CDSCO License for Mammographic x-ray system
Medical Device Information
Intended Use
A mammographic x-ray system is a device intended to be used to produce radiographs of the breast
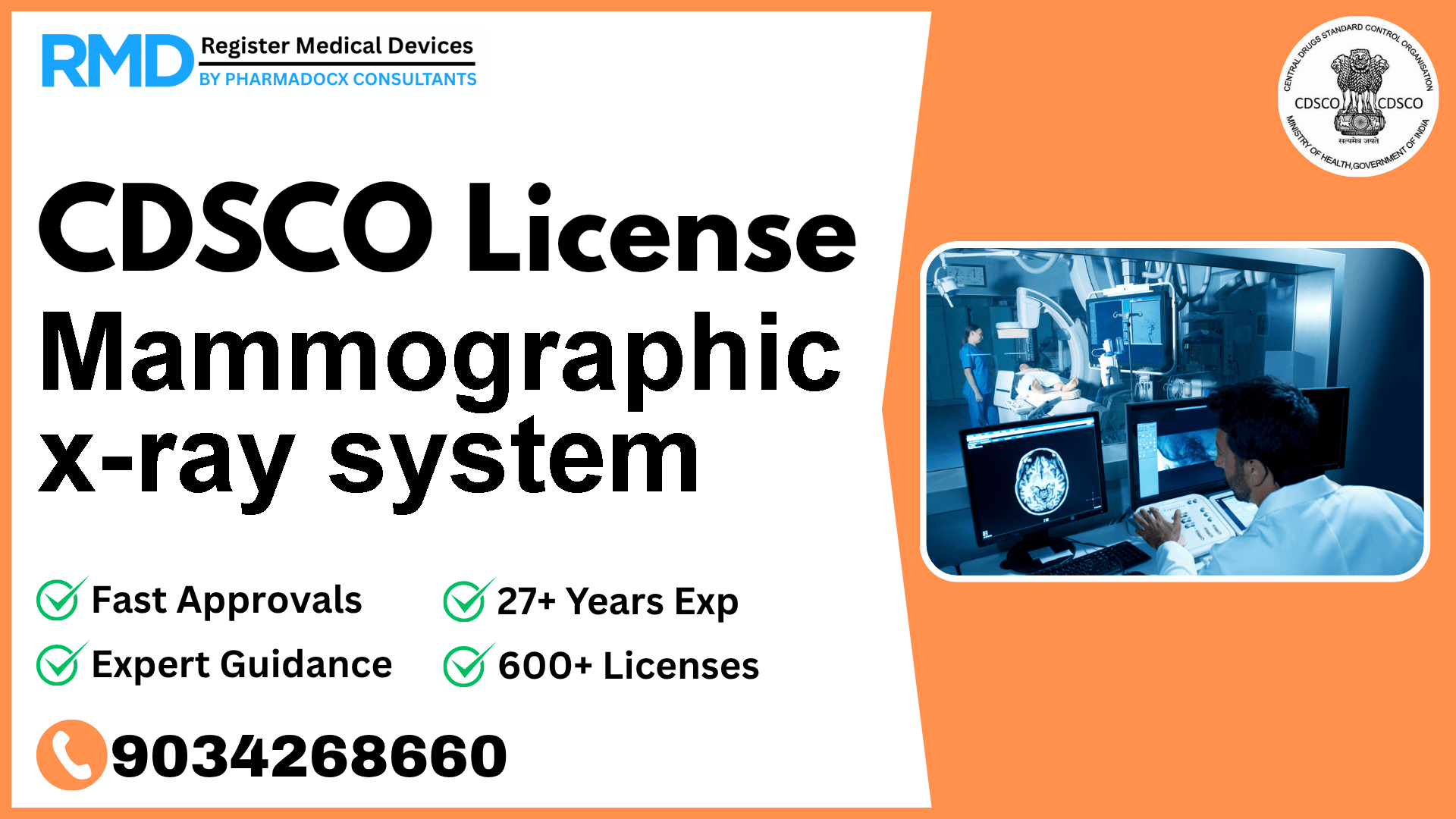
Comprehensive Guide to CDSCO Licensing for Mammographic X-Ray Systems (Class C)
Mammographic x-ray systems play a critical role in breast cancer screening and diagnosis by producing high-quality radiographs of the breast tissue. As interventional radiology devices classified as Class C under the CDSCO regulatory framework, they require thorough evaluation and licensing before entry into the Indian market. With over 25 years of experience assisting 500+ medical device companies, we provide you with a step-by-step, practical guide to obtaining your CDSCO licenses efficiently and compliantly.
Understanding the CDSCO Regulatory Framework for Mammographic X-Ray Systems
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India, including mammographic x-ray systems, under the Medical Device Rules, 2017. These devices fall under Class C due to their medium to high risk, involving ionizing radiation and direct patient interaction.
Compliance ensures patient safety, device performance, and market authorization. According to the notification [29/Misc./03/2020-DC (146)] dated 26.07.2021, mammographic x-ray systems are subject to stringent regulatory scrutiny, including manufacturing and import licensing.
Risk Classification and License Requirements for Mammographic X-Ray Systems
- Risk Class: Class C (Medium to High Risk)
- Applicable Licenses:
- Manufacturing License: MD9 (Form MD7) granted by Central Licensing Authority
- Import License: MD15 (Form MD14) granted by Central Licensing Authority
These licenses assure that the device meets Indian regulatory standards for safety and efficacy.
Manufacturing License Process for Mammographic X-Ray Systems (MD9)
Stepwise Process:
- Test License (Form MD13): Initially, manufacturers must obtain a test license to manufacture the device for testing purposes. This phase typically takes 1.5 to 2 months.
- Laboratory Testing: Devices must undergo testing at CDSCO-approved laboratories to verify compliance with Indian standards and essential principles. Testing Laboratories List provides authorized labs.
- Documentation Preparation: Prepare comprehensive technical documentation including Device Master File, Plant Master File, Risk Management File, and Quality Management System (QMS) documents.
- Application Submission: Apply online via the CDSCO MD Online Portal using Form MD7.
- Regulatory Audit: CDSCO inspectors conduct a facility audit and review the documentation.
- Query Resolution: Address any queries or deficiencies raised during audit or review.
- License Grant: Upon successful compliance, the MD9 manufacturing license is granted.
Timeline:
- Entire process takes approximately 4 to 5 months.
Manufacturing License Documents Required for MD9
- Company Constitution (Incorporation Certificate, MOA/AOA)
- Proof of premises ownership or lease agreement
- Details and qualifications of technical staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) — for detailed device design and manufacturing info (Device Master File Guide)
- Plant Master File (PMF) — details of manufacturing site (Plant Master File Guide)
- Essential Principles Checklist
- Risk Management File complying with ISO 14971 (Risk Management Guide)
- Test Reports from CDSCO-approved labs
- Device labels and Instructions for Use (IFU)
- Quality Management System documents (preferably ISO 13485:2016 certified)
Import License Process for Mammographic X-Ray Systems (MD15)
For importers, the MD15 license is mandatory to bring Class C devices into India.
Stepwise Process:
- Document Preparation: Assemble all required documents including manufacturing license from the country of origin, Free Sale Certificate, and quality certifications.
- Online Application: Submit application through the CDSCO MD Online Portal using Form MD14.
- Review and Queries: CDSCO evaluates documents and may raise queries.
- License Issuance: Upon satisfactory review, import license MD15 is granted.
Timeline:
- Expect approximately 5 to 6 months for processing.
Import License Documents Required (MD15)
- Manufacturing License from country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate or equivalent
- Device Master File
- Plant Master File
- Wholesale License (if applicable)
- Company Constitution Documents
Government Fees and Costs
| License Type | Fee Structure | Approximate Cost (INR) |
|---|---|---|
| MD9 Manufacturing | Rs 50,000 per application + Rs 1,000 per product | Rs 50,000 + Rs 1,000 × number of products |
| MD15 Import | Class C & D: Rs 3,000 per site + Rs 1,500/product | Rs 3,000 + Rs 1,500 × number of products |
Note: Fees are subject to revision; always confirm on CDSCO MD Online Portal.
Timeline and Processing Duration Summary
- MD9 Manufacturing License: 4–5 months including test license and audit
- MD15 Import License: 5–6 months
- Test license (MD13) issuance: 1.5–2 months before MD9
Common Challenges and Practical Solutions
Challenge 1: Delays in Laboratory Testing
- Solution: Engage with accredited testing labs early and confirm sample requirements to avoid backlogs.
Challenge 2: Incomplete or Non-Compliant Documentation
- Solution: Use expert-prepared templates for Device Master File and Risk Management Files. Review using the Device Master File Guide.
Challenge 3: Audit Non-Conformities
- Solution: Conduct internal pre-audit assessments and ensure all QMS processes align with ISO 13485 standards.
Challenge 4: Navigating Regulatory Queries
- Solution: Maintain timely communication with CDSCO authorities and provide thorough, documented responses.
Expert Consultation and Support
With extensive experience navigating CDSCO’s regulatory landscape for Class C devices like mammographic x-ray systems, we offer tailored support including:
- Comprehensive document preparation
- Test license application assistance
- Coordination with notified bodies and laboratories
- Pre-audit readiness assessments
- Query resolution and follow-up
Our proactive approach has helped over 500 companies launch their devices successfully in India.
Getting Started with Your CDSCO License Application
- Assess Device Classification: Confirm your device is Class C using the Medical Device Classification guide.
- Initiate Test License Application (MD13): File through the CDSCO MD Online Portal to start manufacturing for testing.
- Plan for Device Testing: Coordinate with CDSCO-approved labs early to schedule testing.
- Prepare Key Documents: Use our guides to develop your Device Master File, Plant Master File, and Risk Management documentation.
- Submit MD9 Application: Once testing is complete, submit Form MD7 via the online portal.
- Prepare for Audit: Arrange your QMS and facility for inspection.
- Monitor Application Progress: Respond promptly to any CDSCO queries.
- Apply for Import License (MD15) if applicable: Assemble import documents and file Form MD14.
By following these practical steps and leveraging expert guidance, you can streamline your path to obtain the required CDSCO licenses for mammographic x-ray systems and successfully enter the Indian market.
For personalized support, reach out to our regulatory experts who have a proven track record in medical device compliance.
For more detailed insights, explore our dedicated guides on MD9 License, Import License, and Risk Management.