CDSCO License for Manual radionuclide applicator system
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A manual radionuclide applicator system is a manually operated device intended to apply a radionuclide source into the body or to the surface of the body for radiation therapy.
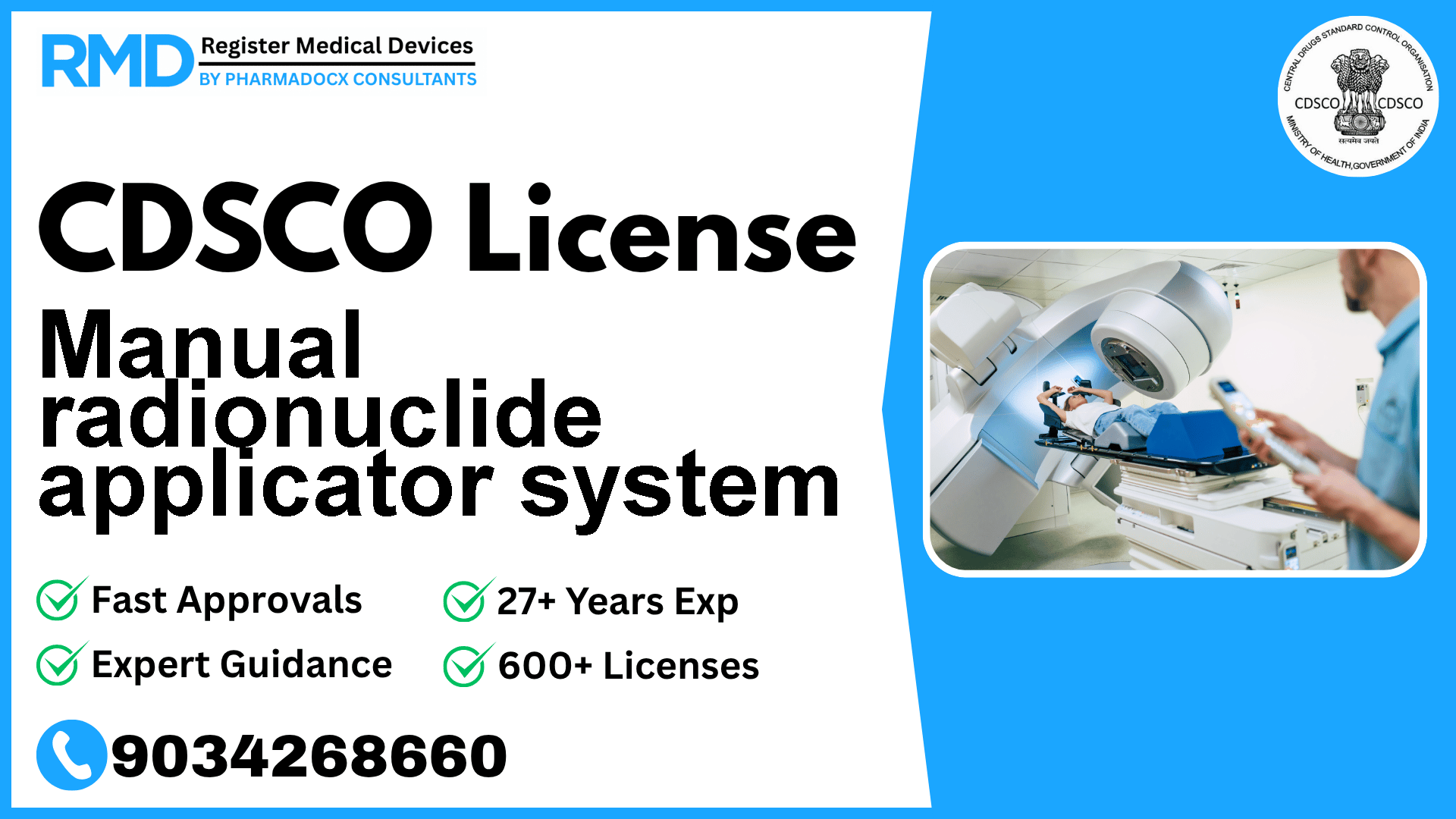
Introduction to Manual Radionuclide Applicator System and Regulatory Importance
A Manual Radionuclide Applicator System is a specialized medical device used in radiotherapy to manually apply a radionuclide source directly to or into the body for therapeutic radiation treatment. Given its critical role in cancer therapy and radiation safety, regulatory oversight by the Central Drugs Standard Control Organization (CDSCO) ensures that these devices meet stringent quality, safety, and efficacy standards before entering the Indian market.
With our 25+ years of experience assisting over 500 medical device companies, we understand the nuances and regulatory pathways involved in obtaining the necessary CDSCO license for this Class A medical device. This guide will walk you through the entire process, from classification to license issuance, with practical tips tailored to the Manual Radionuclide Applicator System.
CDSCO Regulatory Framework for Manual Radionuclide Applicator System
In India, medical devices are regulated under the Medical Device Rules (MDR) 2017, enforced by CDSCO. The Manual Radionuclide Applicator System falls under the Radiotherapy category and is classified as a Class A (low risk) device as per CDSCO's risk classification framework.
The regulatory framework mandates manufacturers to obtain a manufacturing license (MD5) from the State Licensing Authority before commercializing the device. The process involves compliance with quality management systems, product testing, and audits to ensure adherence to the Essential Principles of Medical Device Safety and Performance.
For detailed classification, refer to our Medical Device Classification guide.
Risk Classification and License Requirements for Class A Devices
Class A devices, including the Manual Radionuclide Applicator System, are considered low risk. Therefore, the applicable license is the MD5 Manufacturing License, obtained through Form MD3 via the State Licensing Authority.
Key points:
- Risk Class: A
- License Type: MD5 Manufacturing License
- Regulatory Authority: State Licensing Authority
- Total Processing Time: Approximately 3-4 months
- Fees: Rs 5,000 per application + Rs 500 per product
Class A devices also require a Test License (MD13) initially to facilitate product testing before the manufacturing license can be granted.
Manufacturing License Process (MD5) for Manual Radionuclide Applicator System
The MD5 license process for Class A devices follows these major steps:
Apply for Test License (Form MD13): This license permits you to manufacture the device for testing purposes. It generally takes 1.5 to 2 months to be granted.
Product Testing: Get your Manual Radionuclide Applicator System tested at a CDSCO-approved laboratory. Testing laboratories can be found on the CDSCO Testing Laboratories List.
Documentation Preparation: Prepare comprehensive technical documentation, including Device Master File, Plant Master File, Risk Management File, Essential Principles Checklist, and Quality Management System (QMS) documents.
Submit MD5 License Application (Form MD3): Submit your application on the CDSCO MD Online Portal.
Audit by Notified Body: An audit of your manufacturing premises and QMS will be conducted by a notified body. You can select one from the Notified Bodies List.
Respond to Queries: Address any clarifications or observations raised by the CDSCO or the notified body promptly.
Grant of MD5 License (Form MD5): Upon satisfactory compliance, the State Licensing Authority will issue the manufacturing license.
For a detailed walkthrough, refer to our MD5 License Guide.
Manufacturing License Documents Required for Manual Radionuclide Applicator System
To ensure a smooth application process, prepare the following documents meticulously:
- Company Constitution Documents: Certificate of Incorporation, Memorandum & Articles of Association
- Proof of Ownership or Lease Agreement for manufacturing premises
- Technical Staff Details: Qualifications and experience certificates of key personnel
- Fire NOC and Pollution Control NOC from relevant authorities
- Device Master File (DMF): Detailed technical and design specifications (Guide)
- Plant Master File (PMF): Information about the manufacturing facility and processes (Guide)
- Essential Principles Checklist confirming compliance with safety and performance standards
- Risk Management File: Documenting risk analysis and mitigation measures (Guide)
- Product Test Reports from CDSCO-approved laboratories
- Labels and Instructions for Use (IFU) compliant with regulatory requirements
- Quality Management System Documents: Usually ISO 13485:2016 certification and supporting SOPs
Attention to detail in these documents prevents delays and reduces queries during the application review.
Import License Process (MD15) for Manual Radionuclide Applicator System
If you are an importer of manual radionuclide applicator systems, you must obtain an MD15 Import License from the Central Licensing Authority.
The import license process involves:
- Document preparation including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate
- Application submission on the CDSCO MD Online Portal
- Departmental review and query resolution
- Grant of import license (Form MD15)
The import license typically takes 5 to 6 months to process due to the extensive documentation and quality verification required.
For full details on import license requirements and processes, review our Import License Guide.
Import License Documents Required
Key documents for import license application include:
- Valid manufacturing license from the country of origin
- Free Sale Certificate
- ISO 13485:2016 certification
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License for distribution in India
- Company Constitution documents
Ensure all documents are attested and translated into English as necessary to avoid processing delays.
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Documentation Preparation | 2 - 3 weeks |
| MD5 License Application | Submission Date |
| Audit by Notified Body | Within 1 month |
| Query Resolution | 2 - 3 weeks |
| Grant of MD5 License | Within 1 week |
Total expected timeline: Approximately 3 to 4 months.
Government Fees and Costs
- Test License (MD13): Rs 5,000 (approximate, varies by state)
- MD5 License Application: Rs 5,000 per application
- Product Fee: Rs 500 per product (Manual Radionuclide Applicator System in this case)
Additional costs may include:
- Fees for notified body audit (varies by body)
- Product testing charges at CDSCO-approved labs
- Consultancy fees if engaging expert support
Planning your budget accordingly helps prevent unexpected financial bottlenecks.
Common Challenges and Solutions
Challenge 1: Delays in product testing due to lab backlogs
- Solution: Engage early with CDSCO-approved labs and pre-book testing slots. Consider labs listed on the CDSCO Testing Laboratories.
Challenge 2: Document discrepancies causing repeated queries
- Solution: Utilize our comprehensive document checklists and templates. Ensure consistency across Device Master File, Plant Master File, and Risk Management File.
Challenge 3: Audit non-conformities related to QMS implementation
- Solution: Implement ISO 13485:2016 QMS early and conduct internal audits before notified body inspection.
Expert Consultation and Support
Navigating the CDSCO licensing process for a Manual Radionuclide Applicator System demands detailed regulatory understanding and meticulous preparation. With over 25 years of experience and 500+ successful license grants, our consultancy offers:
- Tailored regulatory strategy consulting
- Complete documentation preparation and review
- Coordination with notified bodies and testing labs
- Application submission and query handling
Our expert support significantly reduces timelines and uncertainty, enabling faster market entry.
Getting Started with Your CDSCO License Application
Begin your CDSCO licensing journey by:
- Registering on the CDSCO MD Online Portal to create your account.
- Preparing the Test License (Form MD13) application including initial documents.
- Identifying and contacting a CDSCO-approved testing laboratory.
- Initiating development of your Device Master File and Plant Master File.
- Scheduling a preliminary internal audit of your QMS.
Taking these actionable steps now will streamline your application process. For detailed assistance, reach out to our regulatory experts who can guide you through every phase from document preparation to license issuance.
Your Manual Radionuclide Applicator System deserves a smooth entry into the Indian market — partner with experienced professionals to make it happen efficiently and compliantly.