CDSCO License for Non-central circulatory permanent implant manual brachytherapy therapeutic radionuclide source
Medical Device Information
Intended Use
A non-central cardiovascular device which is histocompactible and containing an isotope naturally occurring or produced by an accelerator or a nuclear reactor, intended to be permanently implanted in the body for radiation therapy requiring treatment or symptomatic treatment.
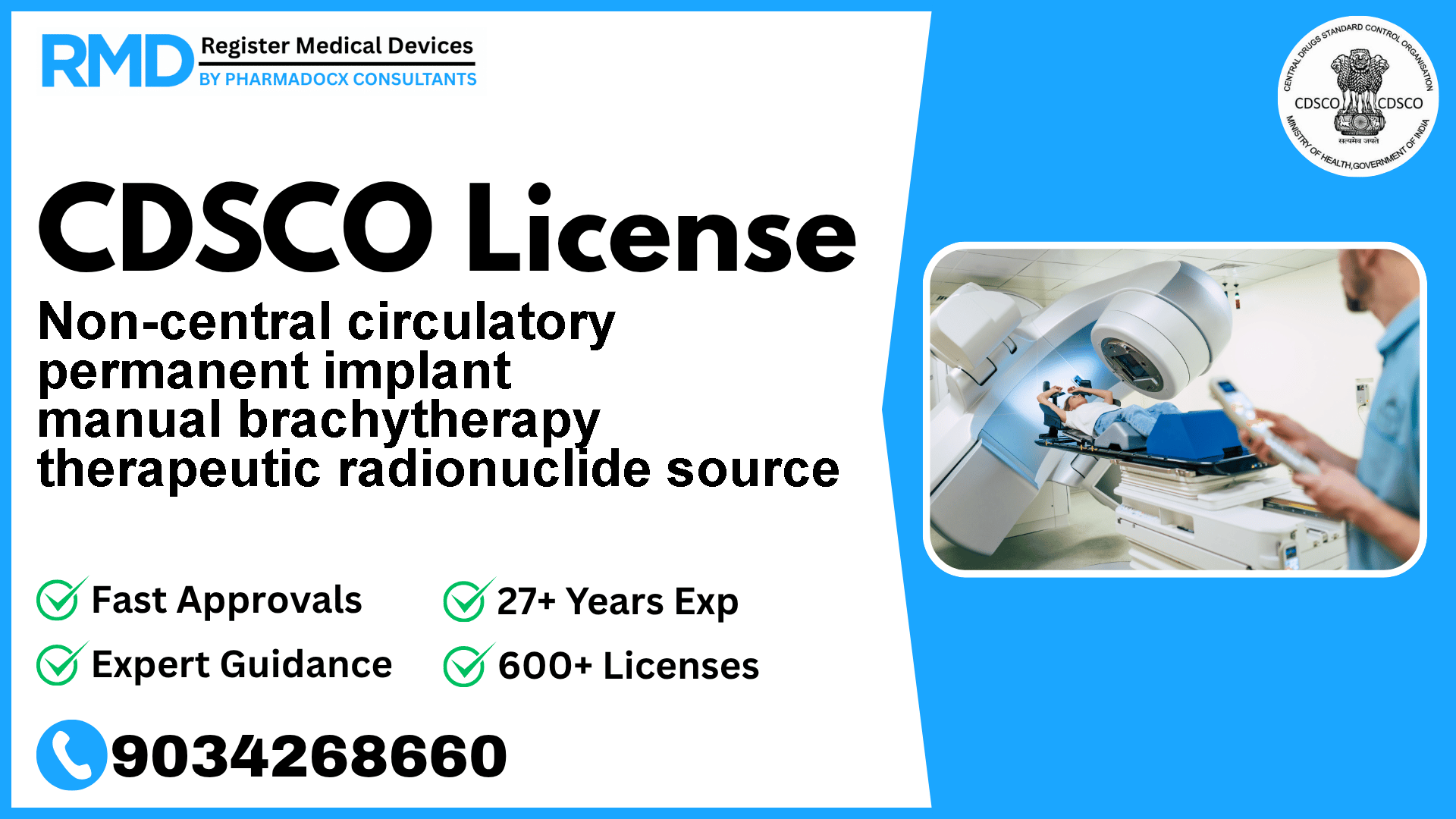
Understanding Non-Central Circulatory Permanent Implant Manual Brachytherapy Therapeutic Radionuclide Source
The Non-central circulatory permanent implant manual brachytherapy therapeutic radionuclide source is a specialized Class C radiotherapy medical device. This device is designed for permanent implantation to deliver localized radiation therapy, using isotopes that are either naturally occurring or produced via accelerators or nuclear reactors. Due to its complex nature and critical therapeutic role, strict regulatory compliance is essential before marketing or manufacturing in India.
CDSCO Regulatory Framework for Radiotherapy Implant Devices
In India, the Central Drugs Standard Control Organization (CDSCO) governs medical device licensing under the Medical Device Rules, 2017. Devices like this radionuclide source fall under Class C, which entails a higher risk classification due to implantation and radiological exposure. Consequently, the licensing authority is centralized, and the approval process is more rigorous to ensure patient safety and device efficacy.
Risk Classification and License Requirements for Class C Devices
This radionuclide source is classified as Class C because of its invasive nature and radiation emission. Under CDSCO regulations:
- Manufacturing License: Requires an MD9 license, granted by the Central Licensing Authority.
- Import License: Requires an MD15 license, also granted centrally.
Both licenses demand comprehensive documentation, product testing, audits, and compliance with essential principles of medical device regulations.
Manufacturing License Process (MD9) for Class C Radiotherapy Implants
Obtaining an MD9 manufacturing license involves multiple structured steps:
- Test License Application (Form MD13): Before a full manufacturing license, manufacturers must apply for a test license on Form MD13. This allows limited-scale manufacturing for testing purposes.
- Product Testing: The device must undergo rigorous testing from CDSCO-approved laboratories to verify safety, radiation containment, biocompatibility, and functionality. You can find the list of approved testing facilities on the CDSCO Testing Laboratories page.
- Document Preparation: Compile all essential documents, including the Device Master File, Plant Master File, risk management documentation, and QMS certifications.
- Application Submission (Form MD7): Submit the MD9 application through the CDSCO MD Online Portal with all required documents.
- Inspection & Audit: CDSCO officials will conduct onsite audits to verify compliance with Good Manufacturing Practices (GMP), quality systems, and facility adequacy.
- Query Resolution: Address any queries or observations raised by CDSCO promptly.
- License Grant: Upon satisfactory review, the license is issued on Form MD9.
Timeline
- Test License Processing: Approximately 1.5 to 2 months.
- Product Testing: Typically 1 to 1.5 months depending on lab schedules.
- MD9 Application Processing & Audit: 2 to 3 months.
Total estimated duration: 4 to 5 months.
Costs
- Government Fees: Rs 50,000 per MD9 application.
- Product Fee: Rs 1,000 per product.
- Testing and Audit Costs: Vary depending on lab and notified body charges.
Manufacturing License Documents Required for MD9
Key documents include:
- Company Constitution/Registration Certificate
- Proof of ownership or lease agreement of manufacturing premises
- Details and qualifications of technical staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Comprehensive device specifications, design, manufacturing process, and safety data. Refer to our detailed Device Master File guide.
- Plant Master File (PMF): Details of manufacturing facilities and quality systems. See our Plant Master File guide.
- Essential Principles Checklist confirming compliance with CDSCO regulations
- Risk Management File detailing hazard analysis and mitigation strategies. Learn more about medical device risk management.
- Test Reports from CDSCO-recognized laboratories
- Labels and Instructions for Use (IFU)
- Quality Management System (QMS) certificates such as ISO 13485:2016
Import License Process (MD15) for Class C Radiotherapy Devices
Importers must apply for an MD15 license to legally bring this device into India. The process includes:
- Document Preparation: Assemble documents such as the manufacturing license, Free Sale Certificate, ISO certification, CE certificate (if applicable), and master files.
- Application Submission: Submit Form MD14 for the MD15 license through the CDSCO MD Online Portal.
- Official Review: CDSCO reviews the application and may request clarifications.
- License Grant: Upon compliance verification, the MD15 license is issued.
Timeline
The import licensing process generally takes about 5 to 6 months.
Costs
- Government Fees:
- Class C & D devices: Rs 3,00,000 per site
- Product Fee: Rs 1,50,000 per product
Import License Documents Required
- Valid Manufacturing License (MD9) from the country of manufacture
- Free Sale Certificate or equivalent from the exporting country
- ISO 13485:2016 certificate
- CE certificate or equivalent international certification
- Device Master File and Plant Master File
- Wholesale license from the importer
- Company Constitution and registration certificates
Common Challenges and Practical Solutions
Manufacturers and importers often face challenges such as:
- Delays in Product Testing: Government-approved labs may have backlogs. Plan testing well in advance and consider multiple labs from the CDSCO Testing Laboratories list.
- Incomplete Documentation: Missing or inconsistent files can delay audits. Use checklists and seek expert review before submission.
- Audit Non-Compliance: Prepare for audits by aligning manufacturing processes with GMP and having QMS well documented.
- Query Resolution Delays: Respond promptly to CDSCO queries with clear, comprehensive answers.
Our real-world experience shows that proactive planning, early engagement with CDSCO, and thorough internal audits reduce approval times significantly.
Expert Consultation and Support
With over 25 years and 500+ successful CDSCO license applications, we offer end-to-end support:
- Gap analysis for compliance readiness
- Documentation drafting and review (DMF, PMF, Risk Management Files)
- Coordination with notified bodies and testing labs
- Application preparation and submission via the CDSCO MD Online Portal
- Audit preparation and representation
- Post-approval compliance support
Getting Started with Your CDSCO License Application
- Classify Your Device: Confirm your device’s Class C status and regulatory pathway using the Medical Device Classification guide.
- Initiate Test License: Apply for the MD13 test license to begin limited manufacturing and product testing.
- Plan Testing Early: Contact CDSCO-approved labs to schedule testing to avoid bottlenecks.
- Prepare Documentation: Develop Device and Plant Master Files, Risk Management Files, and gather all statutory certificates.
- Submit MD9 Application: Once testing is complete and documentation is ready, apply for the MD9 manufacturing license.
- Coordinate Audits: Engage with CDSCO auditors and promptly address any observations.
- Apply for MD15 Import License: If importing, prepare the necessary documents and submit MD15 application.
Embarking on this regulatory journey requires diligence and expertise. Partnering with experienced consultants can streamline your approval process, ensuring timely market access for your innovative radiotherapy implant device.