CDSCO License for Non-central circulatory remote after loading brachytherapy therapeutic radionuclide source
Medical Device Information
Intended Use
A device for the non-central circulatory system used as radiation source to deliver a high or low dose rate with an after-loading brachytherapy device designed for radiotherapy which is necessary for treatment and symptomatic therapy, and uses natural radioisotopes or radioisotopes produced by an accelerator or a nuclear reactor.
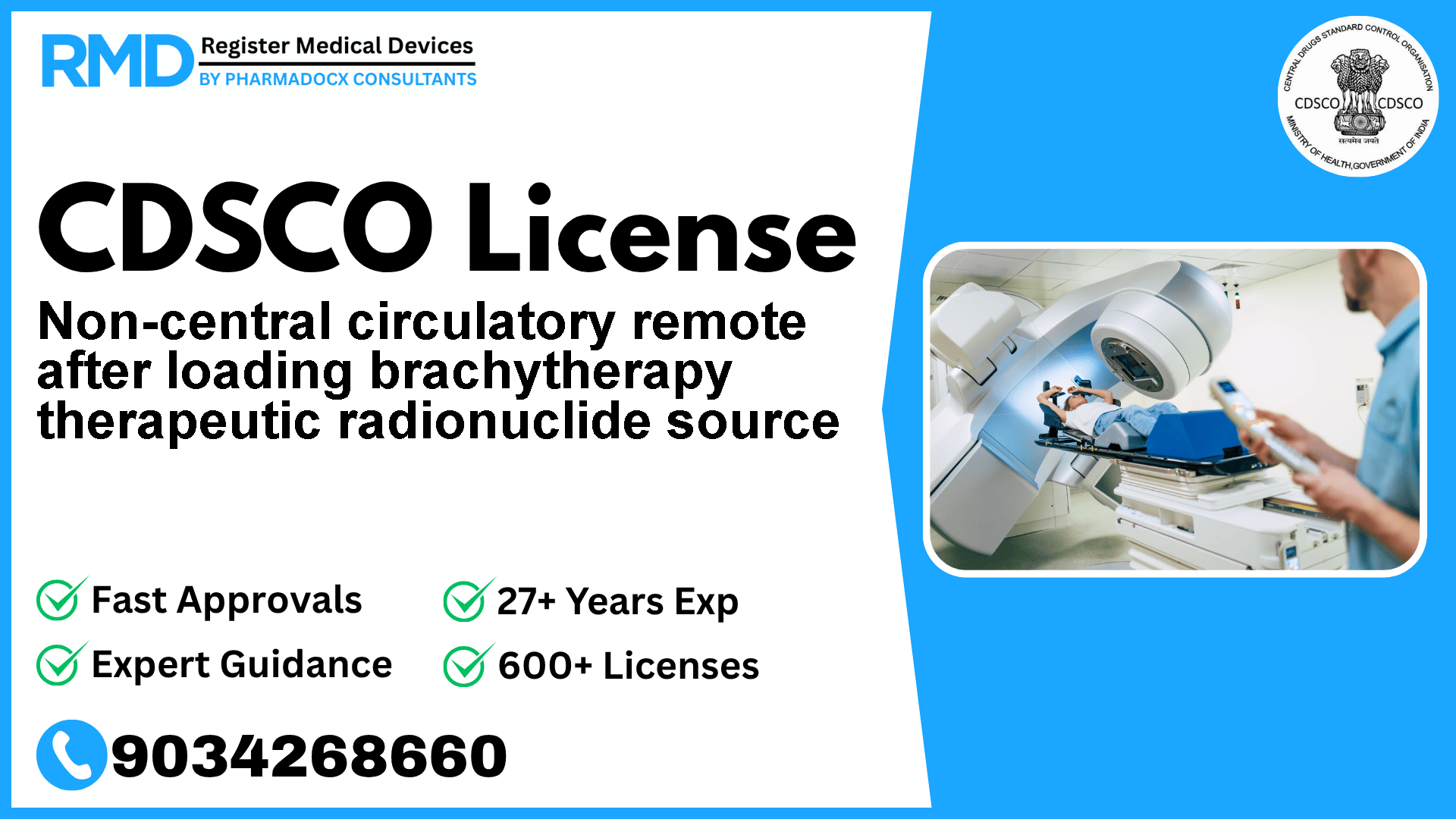
Introduction to Non-Central Circulatory Remote After Loading Brachytherapy Therapeutic Radionuclide Sources
Non-central circulatory remote after loading brachytherapy therapeutic radionuclide sources are specialized medical devices used in radiotherapy to deliver precise doses of radiation. Designed for the non-central circulatory system, these devices utilize natural or accelerator-produced radioisotopes for effective cancer treatment and symptomatic therapy. Given their critical role and inherent risks, regulatory compliance is paramount to ensure patient safety and market access in India.
CDSCO Regulatory Framework for Radiotherapy Devices (Class C)
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India, categorizing them based on risk. Your device falls under Class C, which includes moderate to high-risk devices like radiotherapy sources. Licensing for Class C devices is managed by the Central Licensing Authority through the MD9 license pathway.
This regulatory framework ensures devices meet quality, safety, and efficacy standards before entering the Indian market. The relevant regulation for your device is notified under File No. 29/Misc./03/2020-DC (180) dated 6.8.2021.
Risk Classification and License Requirements for Your Device
- Device Risk Class: C (Moderate to high risk)
- License Type: MD9 Manufacturing License
- Regulatory Authority: Central Licensing Authority, CDSCO
- Application Form: MD7 for manufacturing license
Class C devices require rigorous scrutiny, including testing, documentation, and audits, reflecting their potential impact on human health.
Manufacturing License Process (MD9) for Class C Devices
Obtaining an MD9 license involves several critical steps:
- Test License (MD13): Before full manufacturing license application, manufacturers must obtain a test license on Form MD13, allowing limited manufacturing for testing purposes (duration: 1.5–2 months).
- Product Testing: Submit your device samples to CDSCO-approved testing laboratories for comprehensive evaluation. Access the list of testing laboratories for your device.
- Document Preparation: Compile detailed documentation, including technical files, quality management systems, and risk assessments.
- License Application: Apply for the MD9 manufacturing license using Form MD7 via the CDSCO MD Online Portal.
- CDSCO Inspection: The Central Licensing Authority will conduct an on-site audit to verify compliance.
- Query Resolution: Address any queries or observations raised during audit and document review.
- License Grant: Upon satisfactory compliance, the MD9 license is granted on Form MD9.
For a full guide, refer to our detailed MD9 License Guide.
Manufacturing License Documents Required for MD9
Your application should include:
- Company Constitution Documents: Certificate of incorporation, partnership deed, or LLP agreement
- Proof of Ownership or Lease of Manufacturing Premises: Valid ownership or lease agreement
- Technical Staff Details: Qualification and experience of personnel
- Fire NOC and Pollution Control NOC: From relevant authorities
- Device Master File (DMF): Comprehensive technical dossier; see our Device Master File Guide
- Plant Master File (PMF): Details of manufacturing facility; reference our Plant Master File Guide
- Essential Principles Checklist: Demonstrating compliance with CDSCO regulations
- Risk Management File: Following ISO 14971 principles; consult our Risk Management resource
- Test Reports: From government-approved labs
- Labeling and Instructions For Use (IFU): In English and regional languages
- Quality Management System (QMS) Documents: ISO 13485:2016 certification, SOPs, CAPA, etc.
Ensuring completeness and accuracy in documentation expedites the approval process.
Import License Process (MD15) for Radiotherapy Devices
If you are an importer of this device, an MD15 Import License from the Central Licensing Authority is mandatory. The process includes:
- Documentation Preparation: Including existing manufacturing license, free sale certificate, ISO 13485:2016, CE certificate, Device and Plant Master Files.
- Application Submission: Using Form MD14 on the CDSCO MD Online Portal.
- Query Resolution: Address CDSCO’s observations as required.
- License Grant: Issuance of MD15 license.
Typically, the import license process takes 5–6 months. For detailed guidance, see our Import License Guide.
Import License Documents Required
- Valid manufacturing license (MD9 for your device)
- Free Sale Certificate from country of origin
- ISO 13485:2016 Certificate
- CE Certificate or equivalent
- Device Master File and Plant Master File
- Wholesale Drug License (if applicable)
- Company incorporation documents
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Documentation Preparation | 1 – 2 months (parallel) |
| MD9 License Application | 1 month |
| CDSCO Audit & Inspection | 1 month |
| Query Resolution & License Grant | 0.5 – 1 month |
Total estimated duration: 4 to 5 months for manufacturing license.
Government Fees and Costs
| License Type | Application Fee (INR) | Per Product Fee (INR) |
|---|---|---|
| MD9 Manufacturing | 50,000 | 1,000 |
| MD13 Test License | 5,000 | 500 |
Additional costs include:
- Testing laboratory fees (variable)
- Notified body audit charges
- Consultant fees (if applicable)
Budgeting accurately for these fees helps avoid delays.
Common Challenges and Solutions
Challenge: Delays in product testing due to limited government-approved labs.
Solution: Plan testing early and engage with certified labs listed on the CDSCO website.
Challenge: Insufficient documentation completeness.
Solution: Use checklists and consult expert guidance to ensure all dossiers are thorough.
Challenge: Audit non-compliance due to facility gaps.
Solution: Conduct internal audits and gap assessments before CDSCO inspection.
Challenge: Query management delays.
Solution: Assign dedicated regulatory personnel for prompt response.
Expert Consultation and Support
With over 25 years of experience and successful support to 500+ companies, we specialize in CDSCO licensing for complex devices like your Class C brachytherapy source. Our services include:
- End-to-end license application preparation
- Documentation drafting and review
- Coordination with CDSCO and notified bodies
- Pre-audit readiness and gap analysis
Reach out early in your product development cycle to streamline approval.
Getting Started with Your CDSCO License Application
- Assess Device Risk and Confirm Classification: Verify your device’s Class C status using resources like our Medical Device Classification guide.
- Initiate Test License Application (MD13): Apply through the CDSCO MD Online Portal.
- Engage with Approved Testing Laboratories: Schedule product testing early.
- Prepare Comprehensive Documentation: Develop your Device Master File, Plant Master File, Risk Management File, and QMS documents.
- Plan for CDSCO Audit: Ensure your manufacturing facility and processes comply with regulatory standards.
- Submit MD9 License Application: File Form MD7 after successful testing and test license approval.
- Respond to Queries Promptly: Maintain open communication with CDSCO.
Taking these steps systematically will position your non-central circulatory remote after loading brachytherapy therapeutic radionuclide source for timely CDSCO license approval and successful entry into the Indian market.