CDSCO License for Non-central circulatory remote after loading brachytherapy therapeutic radionuclide system
Medical Device Information
Intended Use
A device that places a radiation source temporarily at the treatment site in the non-central circulatory system for providing a required radiation dose during radiotherapy. This device equips a remotely controlled radiation source transporter.
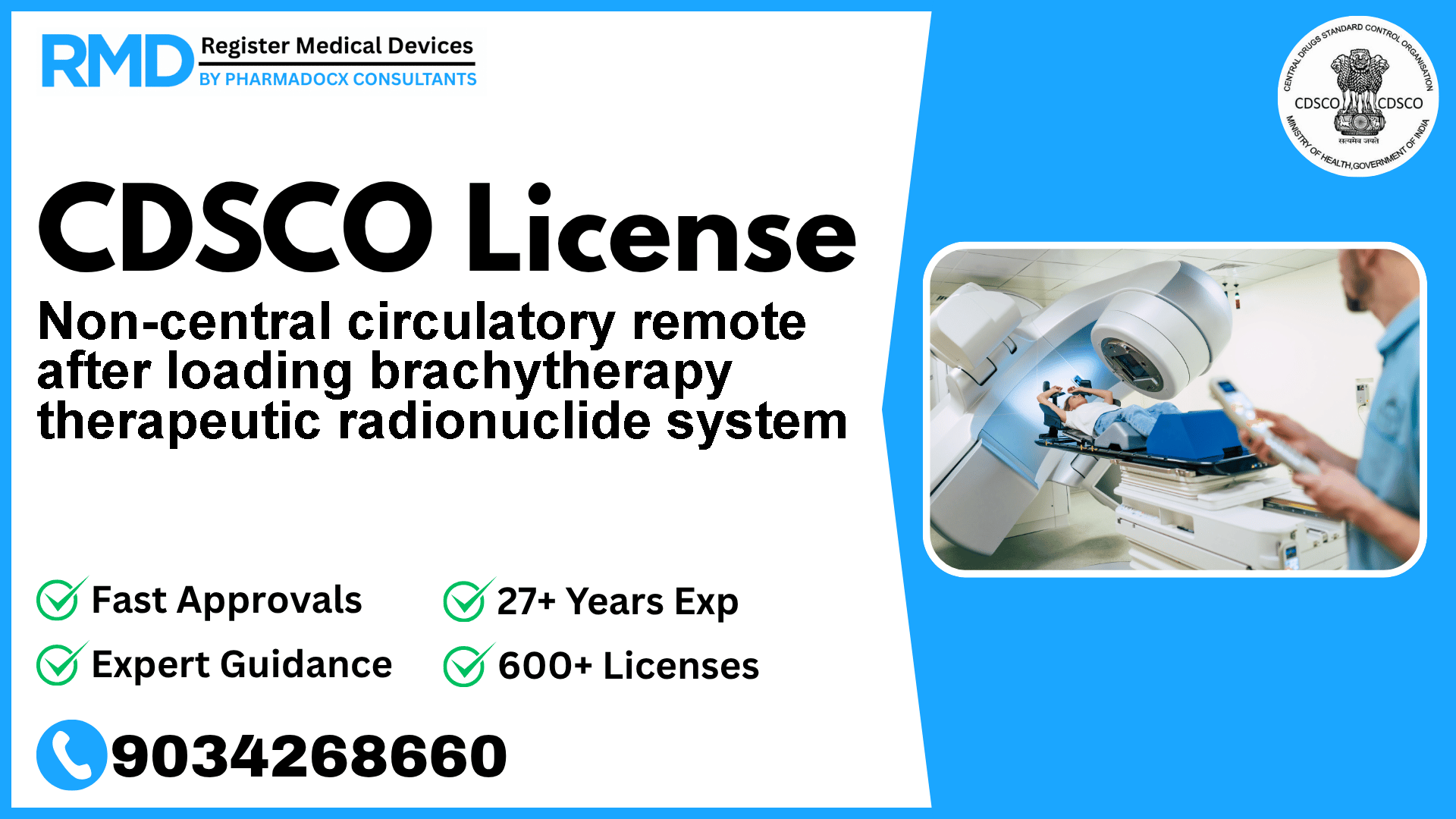
Comprehensive Guide to CDSCO Licensing for Non-Central Circulatory Remote After Loading Brachytherapy Therapeutic Radionuclide System
The non-central circulatory remote after loading brachytherapy therapeutic radionuclide system is a sophisticated Class C radiotherapy device designed to deliver targeted radiation doses by temporarily placing a radiation source at the treatment site within the non-central circulatory system. Equipped with a remotely controlled radiation source transporter, this device plays a critical role in cancer treatment protocols.
Given its complexity and direct patient impact, obtaining the proper regulatory approvals from the Central Drugs Standard Control Organization (CDSCO) is essential for manufacturers and importers to legally market this device in India. With over 25 years of experience and having supported 500+ companies, we provide you with a detailed, step-by-step guide to the CDSCO licensing process specifically tailored to this device category and risk classification.
CDSCO Regulatory Framework for Radiotherapy Devices like Brachytherapy Systems
The CDSCO regulates all medical devices under the Medical Device Rules, 2017, classifying devices based on inherent risk. Radiotherapy devices such as the non-central circulatory remote after loading brachytherapy system fall under Class C due to their moderate to high risk involving radiation exposure and remote control mechanisms. The licensing authority for Class C devices is the Central Licensing Authority (CLA) under CDSCO.
Risk Classification and License Requirements for Class C Devices
As a Class C device, the non-central circulatory remote after loading brachytherapy system requires the stringent MD9 manufacturing license. This license ensures that manufacturing practices, device safety, and quality controls meet national and international standards.
- License Type: MD9 (Application Form MD7)
- Issuing Authority: Central Licensing Authority, CDSCO
- Total Process Duration: Approximately 4-5 months
- Notification Reference: File No. 29/Misc./03/2020-DC (180), dated 6.8.2021
For complete details on classification and regulatory framework, refer to our Medical Device Classification guide.
Manufacturing License Process (MD9) for Class C Radiotherapy Devices
The MD9 license process involves several stages:
- Test License (Form MD13): Initiate by obtaining the test license, valid for 3 months (extendable), to conduct performance and safety testing of your device. This takes approximately 1.5-2 months.
- Product Testing: Submit your device to CDSCO-approved government testing laboratories. Testing includes safety, radiation leakage, and functional performance, critical for radiotherapy devices. Explore the list of Testing Laboratories recognized by CDSCO.
- Document Preparation: Compile detailed technical documentation including Device Master File (DMF), Plant Master File (PMF), Essential Principles Checklist, Risk Management File, and Quality Management System (QMS) records.
- Application Submission: File the MD9 manufacturing license application on the CDSCO MD Online Portal using Form MD7.
- Audit and Inspection: CDSCO inspectors conduct a thorough audit of your manufacturing facility and documentation.
- Queries and Resolution: Address any observations or queries raised during the audit.
- License Grant: Upon successful compliance, CDSCO issues the MD9 manufacturing license (Form MD9).
Manufacturing License Documents Required for MD9
For Class C devices like the brachytherapy system, CDSCO mandates comprehensive documentation:
- Company Constitution: Incorporation certificates and legal entity proof.
- Proof of Ownership or Lease Agreement: For manufacturing premises.
- Technical Staff Qualification Documents: Engineers, quality managers, and radiation safety officers.
- Fire NOC and Pollution Control Certificate: Compliance with environmental and safety norms.
- Device Master File (DMF): Detailed device design, specifications, and manufacturing processes. Our Device Master File guide can assist in crafting this critical document.
- Plant Master File (PMF): Information on manufacturing infrastructure and equipment. Refer to our Plant Master File guide for best practices.
- Essential Principles Checklist: Demonstrating compliance with applicable standards.
- Risk Management File: Documented risk analysis and mitigation strategies consistent with ISO 14971. Learn more about risk management here.
- Product Test Reports: From CDSCO-approved labs verifying device safety and efficacy.
- Labels and Instructions for Use (IFU): Clear, compliant labeling and user manuals.
- Quality Management System Documents: ISO 13485 certification and related SOPs.
Import License Process (MD15) for Class C Radiotherapy Devices
For importers, the MD15 license is mandatory for marketing this device in India. The process includes:
- Document Preparation: Compile manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device and Plant Master Files, Wholesale License, and company constitution.
- Application Submission: Submit Form MD14 application through the CDSCO MD Online Portal.
- Queries Resolution: Respond to any department inquiries.
- License Grant: Typically takes 5-6 months post submission.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Mark Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale Drug License
- Company Constitution
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 1 - 1.5 months |
| Document Preparation | 1 month |
| Application Review & Audit | 1.5 - 2 months |
| Total for MD9 License | Approx 4-5 months |
| Import License (MD15) | 5-6 months |
Government Fees and Costs
- MD9 Manufacturing License: ₹50,000 per application + ₹1,000 per product
- Test License (MD13): Fees nominal but varies by state
- Import License (MD15) Fees:
- Class C & D: 1,500 per product
Additional costs include testing fees at government labs, audit fees (if applicable), and consultancy charges if you engage experts.
Common Challenges and Solutions
Challenge: Delays in product testing due to limited government lab slots.
- Solution: Schedule testing well in advance and consider pre-testing at notified private labs if feasible.
Challenge: Incomplete or inconsistent documentation causing audit observations.
- Solution: Utilize standardized templates for DMF, PMF, and QMS. Engage experienced consultants to review documents before submission.
Challenge: Queries from CDSCO taking longer to resolve.
- Solution: Prepare detailed, clear responses and maintain open communication with CDSCO officers.
Challenge: Managing radiation safety compliance.
- Solution: Employ qualified radiation safety officers and maintain thorough radiation monitoring records.
Expert Consultation and Support
Navigating CDSCO licensing for a Class C radiotherapy device requires meticulous planning and regulatory expertise. Our team has guided 500+ manufacturers and importers through the entire lifecycle—from test licensing to final approval—ensuring compliance while minimizing delays.
We offer:
- Customized document preparation support
- Liaison with CDSCO and notified bodies
- Audit preparedness training
- Post-approval compliance and vigilance strategies
Getting Started with Your CDSCO License Application
- Assess Device Classification: Confirm your device falls under Class C as per notification 29/Misc./03/2020-DC (180).
- Register on CDSCO MD Online Portal: Begin your application process by creating an account at the official CDSCO MD Online Portal.
- Apply for Test License (MD13): Initiate testing procedures early to avoid bottlenecks.
- Identify Accredited Testing Labs: Choose from the government-approved Testing Laboratories.
- Prepare Comprehensive Documentation: Draft your Device Master File, Plant Master File, Risk Management files, and Essential Principles Checklist.
- Engage with Notified Bodies: For audit readiness, consult the list of notified bodies to identify suitable auditors.
- Submit Application and Track: Use the CDSCO portal to submit your MD9 application and monitor status updates.
Embarking on this regulatory journey with expert guidance ensures your non-central circulatory remote after loading brachytherapy therapeutic radionuclide system reaches patients safely and compliantly. Contact us today to leverage our 25+ years of CDSCO licensing expertise and streamline your market entry process.