CDSCO License for Non-powered accelerator system table
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A mechanically-operated bed for radiotherapy designed to adjust the patient's posture and immobilize the patient for radiotherapy that uses a medical linear accelerator or non-linear accelerator.
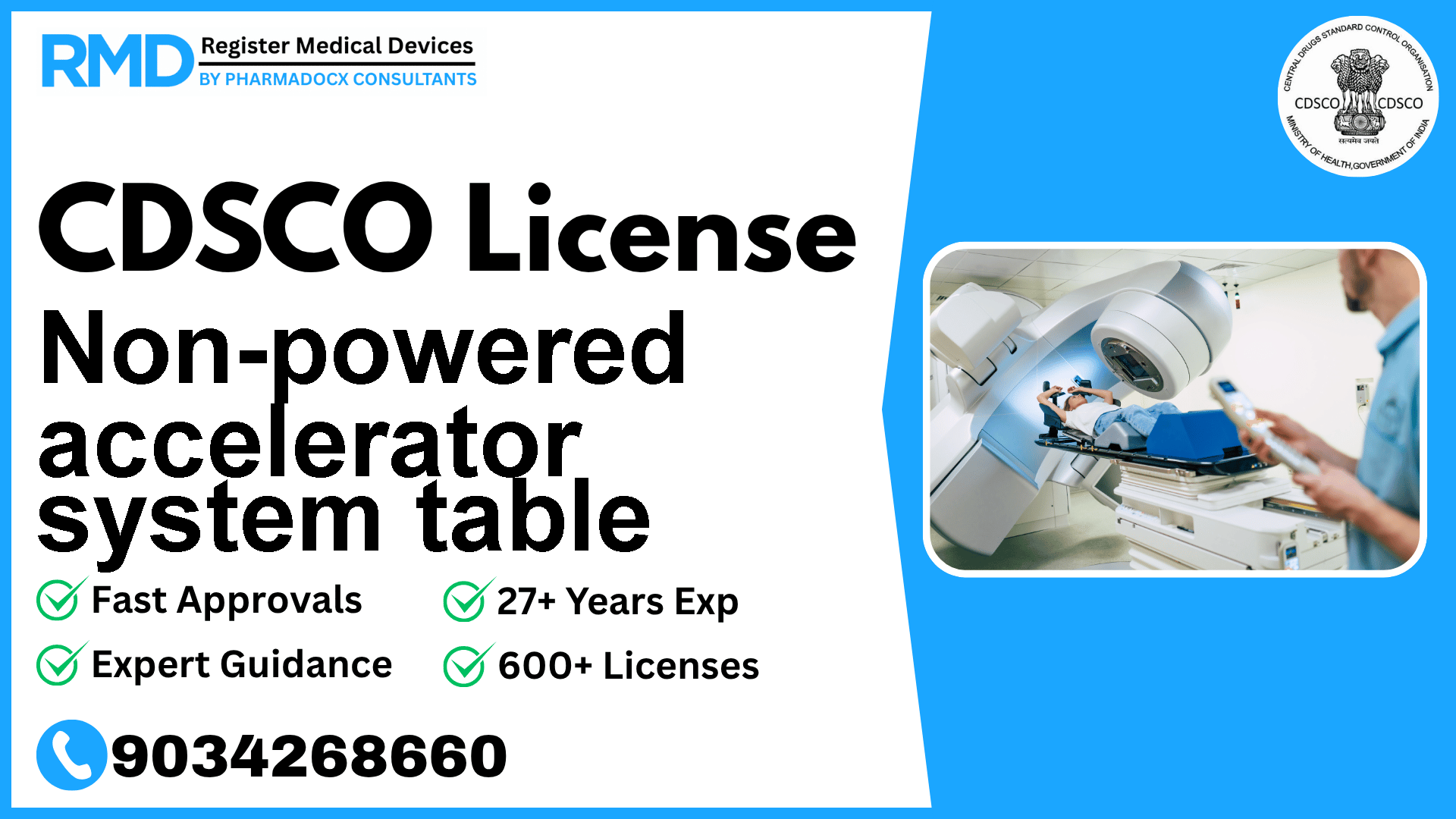
Comprehensive Guide to Obtaining CDSCO License for Non-Powered Accelerator System Table (Class A Device)
Navigating the regulatory landscape for medical devices in India can be complex, especially for specialized equipment such as a non-powered accelerator system table used in radiotherapy. This mechanically-operated bed is designed to adjust and immobilize patients during radiotherapy procedures involving linear or non-linear accelerators, classified under Risk Class A by the CDSCO. With over 25 years of experience assisting more than 500 companies in obtaining CDSCO licenses, we provide you with a detailed roadmap tailored specifically for this device type.
CDSCO Regulatory Framework for Non-Powered Accelerator System Tables
The Central Drugs Standard Control Organization (CDSCO) governs the import, manufacture, and sale of medical devices in India. Since the notification dated 6.8.2021 (File No. 29/Misc./03/2020-DC (180)), this device falls under the radiotherapy category and is classified as Class A, indicating low risk. Compliance with CDSCO regulations ensures that your device meets safety, quality, and performance standards critical for patient care.
Understanding Risk Classification and License Requirements
According to CDSCO's risk-based classification, Class A devices are considered low risk, and their licensing is managed by the respective State Licensing Authority under the MD5 licensing scheme. For your non-powered accelerator system table:
- Device Class: A (Low Risk)
- Applicable License: MD5 (Manufacturing License for Class A/B devices)
- Governing Authority: State Licensing Authority
- Application Form: MD3 for manufacturing license
For more details on medical device classification, refer to our Medical Device Classification guide.
Manufacturing License Process for MD5 (Class A Devices)
The MD5 license process is well-structured but requires careful planning and adherence to timelines:
- Test License Application (Form MD13): First, apply for a test license, which typically takes 1.5 to 2 months to obtain. This license permits product testing from government-approved laboratories.
- Product Testing: Submit the non-powered accelerator system table to CDSCO-recognized testing labs. Testing duration varies but generally completes within 4 to 6 weeks.
- Document Preparation: Compile all required documents meticulously to avoid delays.
- License Application (Form MD3): Submit the manufacturing license application via the CDSCO MD Online Portal.
- Audit by Notified Body: A mandatory audit is conducted by a notified body listed here. Audits ensure compliance with QMS and manufacturing standards.
- Query Resolution: Address any queries or clarifications raised by the licensing authority or notified body promptly.
- License Grant (Form MD5): Upon satisfactory completion of all steps, the MD5 license will be granted.
The entire process typically spans 3 to 4 months, assuming timely submission and audit scheduling.
Manufacturing License Documents Required for Non-Powered Accelerator System Table
Accuracy and completeness in document submission are crucial. For this device, prepare the following:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire Safety No Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File (DMF): Comprehensive technical dossier including design, specifications, and performance data (Device Master File Guide)
- Plant Master File (PMF): Documentation of manufacturing facilities and processes (Plant Master File Guide)
- Essential Principles Checklist demonstrating compliance with safety and performance standards
- Risk Management File highlighting risk identification and mitigation (Risk Management Guide)
- Test Reports from CDSCO-approved laboratories (Testing Laboratories)
- Labels and Instructions for Use (IFU) compliant with CDSCO requirements
- Quality Management System (QMS) Documentation demonstrating adherence to ISO 13485:2016 or equivalent
Import License Process (MD15) for Non-Powered Accelerator System Tables
If you are importing this device, an MD15 import license from the Central Licensing Authority is mandatory. The process takes approximately 5 to 6 months and involves:
- Document preparation including manufacturing license from country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE certificate, and relevant master files.
- Application submission on the CDSCO MD Online Portal
- Query resolution
- License grant on Form MD15
For detailed guidance, consult our Import License Guide.
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 4 - 6 weeks |
| Document Preparation | 2 - 3 weeks |
| License Application Processing | 4 - 6 weeks |
| Notified Body Audit | Scheduled within 1 month post-application |
| Query Resolution and Final Grant | 2 - 3 weeks |
Overall, anticipate 3 to 4 months from start to finish for manufacturing license.
Government Fees and Costs
The fee structure for MD5 licensing (Class A devices) is as follows:
- Application Fee: Rs. 5,000 per application
- Per Product Fee: Rs. 500 per product
Additional costs to consider include:
- Testing laboratory fees (variable depending on test scope)
- Notified body audit fees (varies by body and scope)
- Consultancy and documentation preparation fees if outsourced
Budgeting accurately upfront can prevent unexpected delays.
Common Challenges and Practical Solutions
Challenge 1: Delays in Test License Approval
- Solution: Prepare and submit a complete application with all supporting documents to avoid back-and-forth.
Challenge 2: Incomplete or Non-compliant Documentation
- Solution: Use templates and checklists from experienced consultants to ensure all mandatory documents are included and comply with CDSCO standards.
Challenge 3: Scheduling Notified Body Audits
- Solution: Early engagement with notified bodies listed here helps secure audit slots and avoid bottlenecks.
Challenge 4: Addressing Regulatory Queries
- Solution: Respond promptly and comprehensively to queries with factual, document-backed answers.
Expert Consultation and Support
Given the technical and regulatory complexities, partnering with a seasoned regulatory consultant can significantly streamline the license acquisition process. We have successfully guided over 500 companies through regulatory approvals, ensuring compliance and market readiness. Our expertise includes:
- Comprehensive gap analysis of documentation
- Coordinating with testing labs and notified bodies
- Preparing and submitting applications via the CDSCO MD Online Portal
- Handling communication with CDSCO to resolve queries effectively
Getting Started with Your CDSCO License Application
To initiate your MD5 license application for the non-powered accelerator system table:
- Register on the CDSCO MD Online Portal: Create a company profile and familiarize yourself with the portal interface.
- Prepare Test License Application: Gather all initial documents for Form MD13 and submit to the State Licensing Authority.
- Engage a CDSCO-Approved Testing Laboratory: Select a lab from the official Testing Laboratories list and schedule testing.
- Develop Comprehensive Master Files: Utilize our Device Master File and Plant Master File guides to compile technical documentation.
- Plan for Notified Body Audit: Contact notified bodies early to schedule the mandatory audit.
- Submit Application for Manufacturing License (MD3): Once testing and documentation are complete, apply for the MD5 license.
By following these targeted steps and leveraging expert support, you can confidently navigate the CDSCO licensing process and bring your non-powered accelerator system table to the Indian market efficiently.
For further assistance or customized support, feel free to contact our regulatory experts who specialize in medical device licensing.