CDSCO License for Non-powered remote irradiation therapy table
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
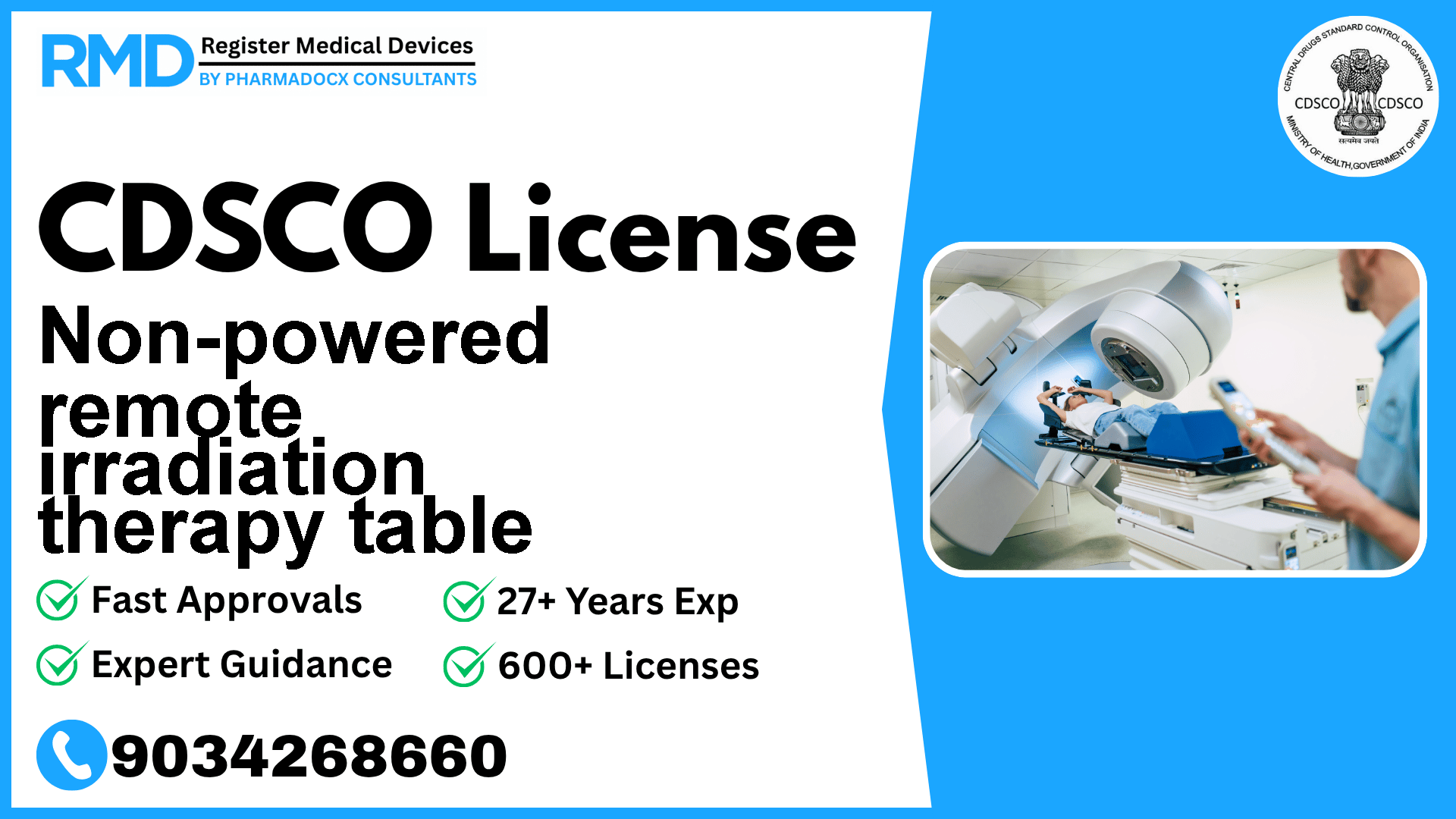
A bed for radiotherapy designed to adjust the patient's posture and immobilize the patient for treatment that uses a remote radionuclide radiotherapy apparatus.
Comprehensive Guide to CDSCO Licensing for Non-Powered Remote Irradiation Therapy Tables (Class A)
Non-powered remote irradiation therapy tables are crucial devices in radiotherapy setups, designed specifically to support and immobilize patients during remote radionuclide radiotherapy treatments. As a Class A medical device, these tables fall under the lowest risk category according to CDSCO regulations but still require stringent compliance with regulatory frameworks to ensure patient safety and market access in India.
With over 25 years of experience supporting 500+ companies in navigating the CDSCO licensing process, we understand the nuances and practical challenges manufacturers face. This guide provides detailed, actionable insights tailored for manufacturers and importers of non-powered remote irradiation therapy tables, helping you secure your CDSCO MD5 manufacturing license efficiently.
CDSCO Regulatory Framework for Non-Powered Remote Irradiation Therapy Tables
The Central Drugs Standard Control Organization (CDSCO) governs medical device regulation in India. Non-powered remote irradiation therapy tables, categorized under radiotherapy devices, are classified as Class A devices – the lowest risk category. Regulatory control ensures that these devices meet quality, safety, and performance standards before entering the Indian market.
The governing notification for this device type is File No. 29/Misc./03/2020-DC (180), dated 6.8.2021, which explicitly includes radiotherapy tables under CDSCO’s purview.
Risk Classification and License Requirements
- Device Risk Class: Class A (Low risk)
- Applicable License: MD5 Manufacturing License (Form MD3)
- Licensing Authority: State Licensing Authority
- Process Overview: Includes obtaining a Test License (Form MD13), product testing from government-approved labs, audit by notified bodies, and final licensing.
Class A devices like the non-powered irradiation table require a simpler regulatory pathway compared to higher-risk devices, but adherence to all compliance steps is mandatory.
Manufacturing License Process (MD5) for Class A Devices
The MD5 license process involves several sequential steps:
Obtain Test License (Form MD13): This prerequisite license allows product testing to be conducted. It generally takes 1.5 to 2 months for approval.
Product Testing: Conduct testing at CDSCO-recognized laboratories to validate compliance with Essential Principles and safety standards. Check the list of testing laboratories for approved facilities.
Document Preparation: Assemble the Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, Quality Management System (QMS) documents, labels, and Instructions for Use (IFU).
Application Submission: Apply for the MD5 license using Form MD3 via the CDSCO MD Online Portal.
Audit by Notified Body: An audit is conducted by a CDSCO-approved notified body to verify compliance. Consult the list of notified bodies for details.
Resolution of Queries: Address any queries or deficiencies raised by the licensing authority or notified body promptly.
Grant of License: Upon satisfactory review, the MD5 license is issued on Form MD5.
This entire process typically spans 3 to 4 months.
Manufacturing License Documents Required
To ensure a smooth application process, prepare the following documents meticulously:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualification Certificates of Technical Staff
- Fire No Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File (DMF) detailing device design, specifications, and manufacturing process. For a comprehensive overview, refer to our Device Master File guide.
- Plant Master File (PMF) describing the manufacturing facility and quality control. Learn more from our Plant Master File guide.
- Essential Principles Checklist confirming compliance with CDSCO regulations
- Risk Management File documenting hazard analysis and risk controls. Our Risk Management guide can help you prepare this effectively.
- Product Test Reports from CDSCO-approved laboratories
- Labels and Instructions for Use (IFU) consistent with regulatory requirements
- Quality Management System (QMS) documentation, preferably ISO 13485 compliant.
Import License Process (MD15) for Non-Powered Remote Irradiation Therapy Tables
If you are an importer seeking to bring this device into India, the MD15 import license is mandatory. This process is managed by the central licensing authority and generally takes 5 to 6 months.
Key steps include:
Document Preparation: Include the manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE certificate, Device Master File, Plant Master File, and wholesale license.
Application Submission: Apply using Form MD14 for the import license via the CDSCO MD Online Portal.
Queries Resolution: Address any regulatory questions promptly.
License Grant: The MD15 license is granted upon satisfactory review.
Fees vary by class and number of products. For Class A devices, the fee is approximately 50 per product.
For detailed guidance, our Import License guide offers step-by-step instructions.
Timeline and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 2 - 3 weeks |
| Document Preparation | 2 - 4 weeks |
| Application Submission (MD5) | Immediate (online) |
| Audit by Notified Body | 3 - 4 weeks |
| Query Resolution | 2 - 3 weeks |
| Total Estimated Time | 3 - 4 months |
Understanding and planning for these timelines can help you schedule your product launch effectively.
Government Fees and Costs
- MD5 Manufacturing License Fees: ₹5000 per application + ₹500 per product
- Test License (MD13) Fees: Included in processing; minimal additional charges may apply for testing
- Product Testing Costs: Variable depending on the testing laboratory and number of tests required
Budgeting accurately will prevent delays and ensure smooth compliance.
Common Challenges and Solutions
Incomplete Documentation: Incomplete or inconsistent files are the most common cause for delays. Ensure all files like DMF, PMF, and Risk Management are comprehensive and up to date.
Test Report Delays: Testing at government-approved labs may have backlogs. Early application for test license and lab appointments is advisable.
Audit Non-compliance: Pre-audit internal checks and mock audits can help prepare your facility and documentation.
Query Resolution Delays: Maintain a dedicated regulatory team or consultant to address queries swiftly.
By preparing proactively, these challenges can be mitigated effectively.
Expert Consultation and Support
Navigating the CDSCO licensing maze requires expertise. With our 25+ years of experience and a track record of 500+ successful approvals, we offer:
- Detailed gap analysis of your current documentation
- End-to-end assistance in document preparation
- Liaison with notified bodies and testing labs
- Audit readiness support
- Timely response management during queries
Partnering with experienced regulatory consultants streamlines your compliance journey, avoids costly errors, and expedites market entry.
Getting Started with Your CDSCO License Application
Here’s a practical roadmap to initiate your license application:
Assess Your Device Classification: Confirm your device is Class A using the Medical Device Classification resource.
Register on CDSCO MD Online Portal: Create your company profile and familiarize yourself with the interface at the CDSCO MD Online Portal.
Prepare Your Test License Application: Gather initial documents and apply for the MD13 test license.
Schedule Product Testing: Engage with CDSCO-recognized labs early to plan your testing timeline.
Compile Master Files and Quality Documentation: Use our guides on Device Master File and Plant Master File preparation.
Engage a Notified Body: Initiate contact to schedule your audit.
Submit MD5 License Application: Once testing and documentation are complete, submit your application and prepare for audit.
Starting early and following these steps methodically will position you for a successful licensing outcome.
Navigating regulatory pathways for devices like non-powered remote irradiation therapy tables can be complex, but with the right approach and expert guidance, you can achieve CDSCO compliance smoothly. Reach out to experienced consultants and leverage proven processes to bring your radiotherapy device to the Indian market confidently and compliantly.