CDSCO License for Non-powered X-rays radiation therapy table
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A bed for radiotherapy designed to adjust the patient's posture and immobilize the patient for treatment that uses an X-ray therapy apparatus.
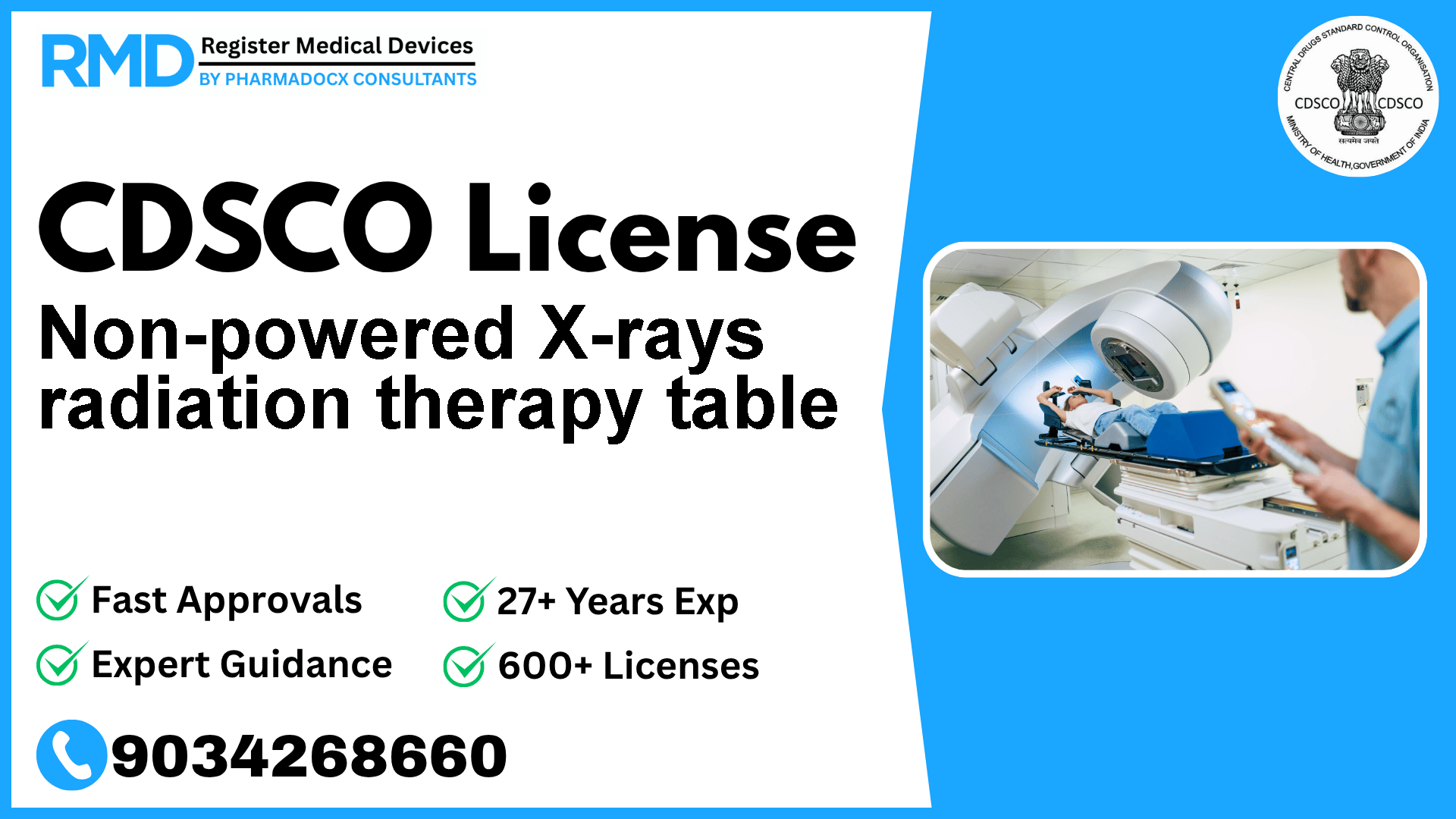
Introduction to Non-powered X-rays Radiation Therapy Table and Its Regulatory Importance
Non-powered X-rays radiation therapy tables serve a critical role in radiotherapy by providing a stable platform to position and immobilize patients during X-ray treatment sessions. As a Class A medical device under CDSCO regulations, this device is considered low risk but requires strict compliance with Indian regulatory standards to ensure patient safety and device efficacy.
Given the specialized use of this device in oncology and radiotherapy, obtaining the appropriate CDSCO license is mandatory for manufacturers aiming to market this product in India. With over 25 years of expertise and having facilitated over 500 successful CDSCO license applications, we provide you with comprehensive guidance tailored to this specific device.
CDSCO Regulatory Framework for Non-powered X-rays Radiation Therapy Table
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices under the Medical Devices Rules, 2017. Non-powered X-rays radiation therapy tables fall under the Radiotherapy category and have been notified under File No. 29/Misc./03/2020-DC (180) dated 6.8.2021.
As a Class A device, the regulatory pathway is overseen by the State Licensing Authority, which evaluates the application for manufacturing licenses under Form MD3 leading to an MD5 license grant.
Risk Classification and License Requirements
This device is classified as Class A (low risk), which impacts the licensing process as follows:
- Manufacturing License: Requires an MD5 license issued by the State Licensing Authority.
- Test License: Prior to the manufacturing license application, obtaining a test license (Form MD13) is mandatory.
- Product Testing: Device samples must undergo testing at CDSCO-approved government laboratories.
Understanding this classification helps streamline your licensing process efficiently and cost-effectively.
Manufacturing License Process (MD5) for Non-powered X-rays Radiation Therapy Table
The manufacturing license for Class A devices like the radiation therapy table requires a multi-step approach:
Apply for Test License (Form MD13): Initiate by submitting the test license application on the CDSCO MD Online Portal. This stage typically takes 1.5 to 2 months.
Product Testing: Submit device samples to CDSCO-recognized testing laboratories. Refer to the list of testing laboratories for options.
Document Preparation: Compile all necessary documents including Device Master File, Plant Master File, risk management file, and quality management system documentation.
Submit MD5 License Application (Form MD3): After successful testing, file your manufacturing license application through the portal.
Audit by Notified Body: A mandatory audit by a notified body ensures compliance with manufacturing and quality standards. You can find the list of notified bodies here.
Resolution of Queries: Address any queries raised by the State Licensing Authority or notified body promptly.
Grant of MD5 License: Upon satisfactory completion, the license is issued on Form MD5.
Manufacturing License Documents Required
For the Non-powered X-rays radiation therapy table, the following documents are essential:
- Company Constitution and Incorporation Certificate
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire NOC and Pollution Control NOC
- Device Master File (DMF) – detailing design, specifications, and performance
- Plant Master File (PMF) – documenting manufacturing processes and facility details
- Essential Principles Checklist confirming compliance with Indian standards
- Risk Management File specific to radiotherapy table use
- Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documentation (ISO 13485:2016 preferred)
For a detailed walkthrough, our comprehensive Device Master File guide and Plant Master File guide provide valuable insights.
Import License Process (MD15) for the Device
If you are an importer rather than a manufacturer, obtaining an MD15 license from the Central Licensing Authority is necessary. The process involves:
- Preparation of detailed documentation including Free Sale Certificate, ISO certification, CE certificate, and existing manufacturing license.
- Application submission on the CDSCO MD Online Portal.
- Resolution of departmental queries.
- License grant within 5-6 months.
Note that no test license is required for import licenses.
Import License Documents Required
For importing the Non-powered X-rays radiation therapy table, ensure the following documents are ready:
- Manufacturing License of the device from the country of origin
- Free Sale Certificate issued by the regulatory authority of the exporting country
- ISO 13485:2016 certification
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License (if applicable)
- Company Constitution and Incorporation certificate
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 1 to 1.5 months |
| Document Preparation | 2 to 4 weeks |
| MD5 License Application (MD3) | 1 month |
| Audit & Query Resolution | 2 to 4 weeks |
| Total Estimated Time | 3 to 4 months |
Planning your submission timeline accordingly is crucial to avoid unnecessary delays.
Government Fees and Costs
For Class A devices like the Non-powered X-rays radiation therapy table, the fee structure is:
- Test License (MD13): Approximately INR 5,000 (varies by state)
- Manufacturing License (MD5): INR 5,000 per application + INR 500 per product
- Additional costs include testing fees at government-approved laboratories and notified body audit charges.
Budgeting for these fees upfront helps prevent last-minute financial bottlenecks.
Common Challenges and Solutions
Challenge 1: Delay in Product Testing
- Solution: Pre-schedule testing with CDSCO-approved labs and ensure sample readiness.
Challenge 2: Incomplete Documentation
- Solution: Use detailed checklists and consult guides such as our MD5 License Guide to ensure all paperwork is in order.
Challenge 3: Audit Non-compliance
- Solution: Conduct internal pre-audits and engage notified bodies early to understand expectations.
Challenge 4: Query Resolution Delays
- Solution: Respond promptly and provide clear, well-supported answers to regulatory queries.
Expert Consultation and Support
Navigating the CDSCO licensing process can be complex, especially for first-time applicants. Our 25+ years of experience in regulatory consulting have helped over 500 companies secure their licenses efficiently. We offer:
- End-to-end application management
- Customized document preparation assistance
- Pre-audit readiness assessments
- Liaison with notified bodies and government authorities
Partnering with experts shortens approval times and reduces compliance risks.
Getting Started with Your CDSCO License Application
To initiate your licensing journey for the Non-powered X-rays radiation therapy table:
- Register on the CDSCO MD Online Portal to access all application forms and submission services.
- Prepare your test license application (Form MD13) and submit samples to approved labs.
- Begin compiling your Device Master File and Plant Master File using our detailed guides.
- Schedule a consultation with experienced regulatory consultants to review your documentation and timeline.
- Plan for the notified body audit by reviewing audit criteria and preparing your manufacturing site.
Starting early and following a structured roadmap ensures you meet all CDSCO requirements and bring your device to the Indian market swiftly and compliantly.
For further details and personalized support, reach out to our regulatory experts who understand the nuances of radiotherapy device licensing in India.