CDSCO License for Non-sterile Surgical forceps
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A surgical or dental device that is used to clamp and sever the cartilage, bone and other hard tissues.
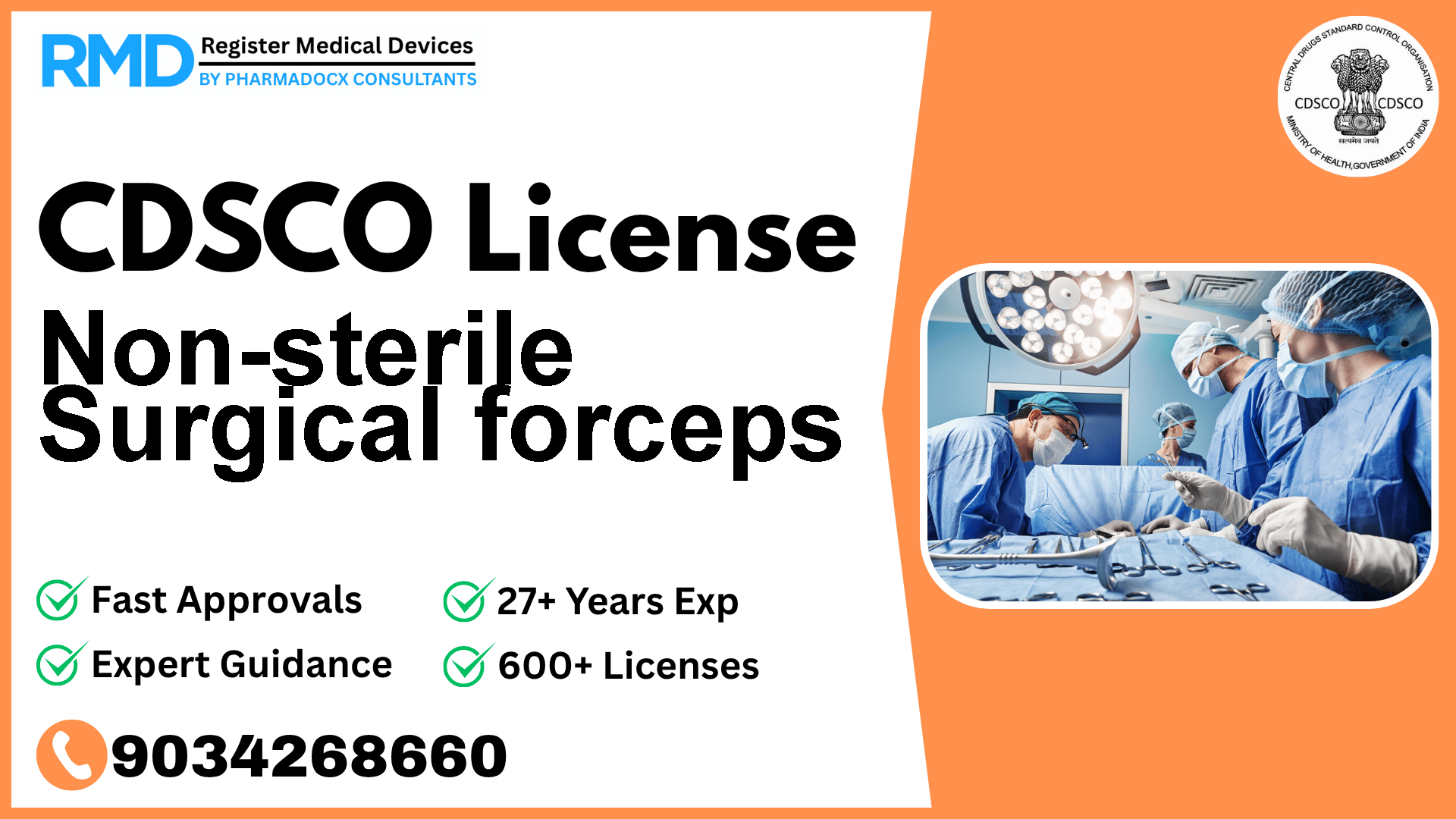
Introduction to Non-sterile Surgical Forceps and Regulatory Importance
Non-sterile surgical forceps are essential medical instruments designed to clamp and sever cartilage, bone, and other hard tissues during surgical or dental procedures. Categorized under operation theatre devices, these forceps play a critical role in ensuring precision and safety in medical interventions. Given their direct interaction with patients in invasive procedures, obtaining regulatory clearance from the Central Drugs Standard Control Organization (CDSCO) is mandatory for manufacturers and importers aiming to supply these devices in the Indian market.
Navigating the CDSCO licensing framework can be complex, especially for first-time applicants. With over 25 years of experience and having successfully assisted more than 500 companies, we provide practical, detailed guidance to streamline your application process for the Non-sterile Surgical Forceps (Risk Class A).
CDSCO Regulatory Framework for Non-sterile Surgical Forceps
Under the Medical Device Rules, 2017, Non-sterile Surgical Forceps are classified as Class A devices — low risk. The regulatory oversight for Class A devices largely falls under the State Licensing Authorities via the MD5 license. The CDSCO MD Online Portal is the official gateway for all application submissions, updates, and tracking.
The key regulatory milestones include obtaining a Test License (Form MD13), product testing by government-approved laboratories, document preparation including Device and Plant Master Files, and finally applying for the Manufacturing License (Form MD3) leading to the issuance of License MD5.
Risk Classification and License Requirements for Class A Devices
Class A devices, including Non-sterile Surgical Forceps, are considered low risk. Consequently, the licensing process is managed at the state level requiring:
- Test License (Form MD13): Permits initial manufacturing for testing purposes.
- Product Testing: Conducted at CDSCO-notified labs.
- Manufacturing License (Form MD3): Application for MD5 license.
This process ensures compliance with safety, quality, and labeling standards essential for market access.
Manufacturing License Process (MD5) for Non-sterile Surgical Forceps
The manufacturing license process for Non-sterile Surgical Forceps involves several stages:
Application for Test License (MD13): Submit through the CDSCO MD Online Portal to obtain permission for trial manufacturing. Expect processing time of 1.5 to 2 months.
Product Testing: After receiving the test license, the device must be tested at government-approved labs. Refer to the list of CDSCO Testing Laboratories for authorized facilities.
Document Preparation: Compile comprehensive documentation including Device Master File, Plant Master File, Risk Management File, Essential Principles Checklist, and Quality Management System documents.
Application for Manufacturing License (Form MD3): Submit the manufacturing license application.
Audit by Notified Body: The state-designated notified body will conduct an audit of manufacturing premises. Check the list of notified bodies for authorized auditors.
Query Resolution: Address any observations or queries raised during the audit or by the licensing authority.
Grant of License (MD5): Upon successful review and audit, the state authority issues the manufacturing license.
Manufacturing License Documents Required
The following documents are essential for the MD5 license application:
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and Qualification Proof of Technical Staff
- Fire NOC and Pollution Control NOC
- Device Master File (DMF) detailing design, specifications, and intended use. Our detailed Device Master File guide can assist in preparation.
- Plant Master File documenting manufacturing processes and quality controls. See our Plant Master File guide for expert tips.
- Essential Principles Checklist confirming compliance with regulatory standards
- Risk Management File highlighting identified risks and mitigation strategies. Learn more about implementing risk management.
- Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, preferably ISO 13485 compliant
Import License Process (MD15) for Non-sterile Surgical Forceps
For importers, the MD15 license is required, granted by the Central Licensing Authority. The process includes:
- Document preparation including Manufacturing License, Free Sale Certificate, ISO 13485:2016, CE Certificate, DMF, PMF, Wholesale License, and Company Constitution.
- Submission of application in Form MD14 via the CDSCO MD Online Portal.
- Resolution of any queries raised.
The import license process generally takes 5-6 months. For detailed information, refer to our comprehensive Import License Guide.
Timeline and Processing Duration
| Step | Estimated Timeframe |
|---|---|
| Test License (MD13) Approval | 1.5 to 2 months |
| Product Testing | 2 to 3 weeks |
| Document Preparation | 3 to 4 weeks |
| Manufacturing License (MD5) Audit | 1 to 1.5 months |
| Query Resolution & Approval | 2 to 4 weeks |
Total Estimated Time: Approximately 3 to 4 months
Government Fees and Costs
- Test License (MD13): Included in MD5 fee structure
- MD5 Manufacturing License:
- Application Fee: Rs. 5,000
- Per Product Fee: Rs. 500
Additional costs include audit fees charged by notified bodies and laboratory testing fees (varies by lab and number of tests).
Common Challenges and Solutions
- Incomplete Documentation: Many applicants submit insufficient technical files. Solution: Use detailed checklists and templates for DMF and PMF.
- Delays in Product Testing: Limited capacity in notified labs can cause bottlenecks. Plan testing early and book slots in advance.
- Audit Non-compliance: Premises or QMS may fail audit due to gaps. Conduct internal pre-audits and staff training.
- Query Resolution Delays: Slow responses can prolong approval. Assign dedicated team members to handle queries promptly.
Expert Consultation and Support
With over two decades of expertise in CDSCO licensing, we provide end-to-end support including gap analysis, document preparation, liaison with authorities, and audit readiness. Our personalized approach has empowered 500+ manufacturers and importers to achieve timely approvals.
Getting Started with Your CDSCO License Application for Non-sterile Surgical Forceps
- Assess Your Product Classification: Confirm Class A status using the Medical Device Classification tool.
- Register on CDSCO MD Online Portal: Create your account to initiate applications.
- Prepare Technical Documentation: Begin compiling Device Master File and Plant Master File with our expert guides.
- Apply for Test License (MD13): Submit your initial application to commence trial manufacturing.
- Schedule Product Testing: Contact CDSCO-approved testing laboratories early.
- Plan for Audit: Select a notified body and arrange internal audits to ensure compliance.
- Submit Manufacturing License Application (MD3): After successful test phase and documentation, proceed with license application.
By following these steps and leveraging our expert consultation, you can confidently navigate the CDSCO licensing process for Non-sterile Surgical Forceps, ensuring smooth market entry and compliance with Indian medical device regulations.