CDSCO License for Nuclear tomography system
Medical Device Information
Intended Use
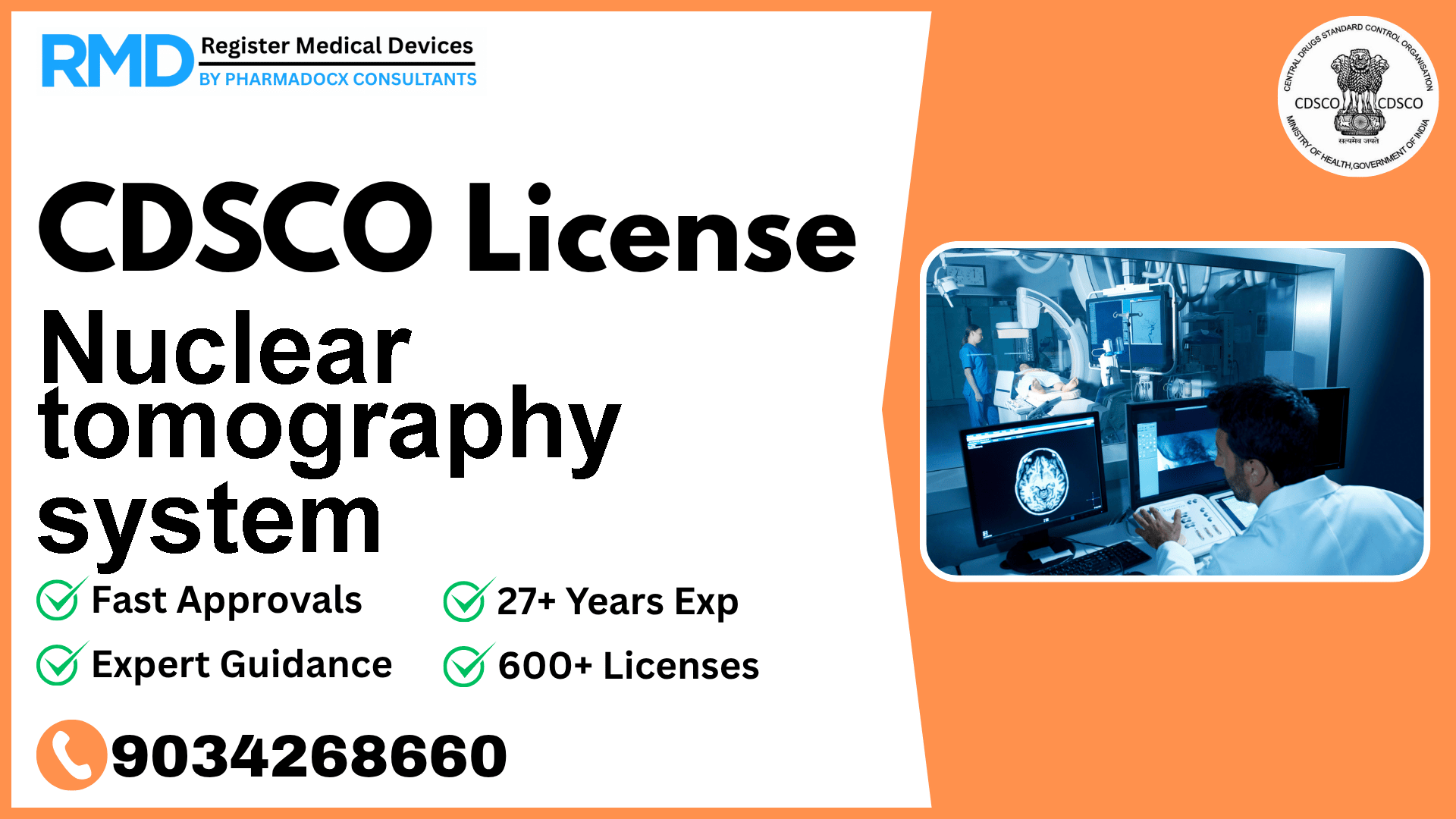
A nuclear tomography system is a device intended to detect nuclear radiation in the body and produce images of a specific cross-sectional plane of the body by blurring or eliminating detail from other planes.
Comprehensive Guide to CDSCO Licensing for Nuclear Tomography Systems (Class C Medical Devices)
As specialists with over 25 years of experience in medical device regulatory consulting, we have successfully guided more than 500 manufacturers and importers through the intricate CDSCO licensing processes. This article provides a detailed, practical roadmap for obtaining regulatory approval in India for Nuclear Tomography Systems, a high-risk Class C medical device classified under Interventional Radiology.
Introduction: Understanding Nuclear Tomography Systems and Regulatory Importance
A Nuclear Tomography System is a sophisticated diagnostic device designed to detect nuclear radiation within the human body and generate detailed cross-sectional images by blurring or eliminating extraneous planes. Its role in interventional radiology is critical for precise diagnostics and treatment planning.
Given its complexity and high-risk nature (Class C), regulatory compliance with the Central Drugs Standard Control Organisation (CDSCO) is mandatory for manufacturing or importing such devices into India. Proper licensing ensures patient safety, device efficacy, and legal market access.
CDSCO Regulatory Framework for Nuclear Tomography Systems
The CDSCO regulates medical devices under the Drugs and Cosmetics Act, 1940, and the Medical Device Rules, 2017. Nuclear Tomography Systems fall under Class C, which are considered moderate to high risk. Licensing for manufacturing and import is overseen by the Central Licensing Authority (CLA) in India.
Manufacturers and importers must comply with strict guidelines, including product testing, quality management system requirements (ISO 13485:2016), and audits by CDSCO inspectors.
Risk Classification and License Requirements for Nuclear Tomography Systems
Class C devices, such as Nuclear Tomography Systems, require an MD9 license for manufacturing and MD15 license for import. This classification demands full compliance with regulatory standards due to potential patient safety implications.
- MD9 License (Manufacturing License - Form MD7)
- MD15 License (Import License - Form MD14)
For detailed classification criteria, manufacturers can refer to the Medical Device Classification guide.
Manufacturing License Process for Nuclear Tomography Systems (MD9 License)
The manufacturing license process for Class C devices like Nuclear Tomography Systems involves several critical steps:
Test License Application (Form MD13): Initially, apply for a test license, which typically takes 1.5 to 2 months to process.
Product Testing: Conduct product testing at government-approved laboratories. Refer to the CDSCO’s Testing Laboratories list to select an accredited lab.
Document Preparation: Compile all required documents meticulously.
Formal Application (Form MD7): Submit the MD9 license application on the CDSCO MD Online Portal.
CDSCO Audit: Undergo an on-site audit by CDSCO inspectors assessing manufacturing facilities, quality systems, and documentation.
Query Resolution: Address any observations or queries raised by the CDSCO or audit team promptly.
License Grant: Upon satisfactory compliance, the MD9 license is granted.
For a detailed understanding, see our MD9 License Guide.
Manufacturing License Documents Required for Nuclear Tomography Systems
The application package must include comprehensive documentation such as:
- Company Constitution and Incorporation Certificate
- Proof of Ownership or Lease of Manufacturing Premises
- Credentials of Qualified Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing the device specifications and design (Device Master File Guide)
- Plant Master File (PMF) describing manufacturing facilities and processes (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with regulatory standards
- Risk Management File demonstrating assessment and mitigation strategies (Risk Management)
- Test Reports from government-approved labs
- Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, including ISO 13485 certification
Import License Process for Nuclear Tomography Systems (MD15 License)
For importers, the MD15 license application follows a distinct but equally rigorous path:
Document Preparation: Compile all necessary documentation.
Application Submission: Submit Form MD14 via the CDSCO MD Online Portal.
Query Resolution: Address any departmental queries promptly.
License Issuance: The CLA grants the MD15 import license.
Notably, an import license does not require a test license (MD13), but requires verified compliance documents including manufacturing licenses from the country of origin.
For a stepwise approach, consult our Import License Guide.
Import License Documents Required for Nuclear Tomography Systems
Key documents include:
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate or Equivalent
- Device Master File and Plant Master File
- Wholesale License for distribution
- Company Constitution and Incorporation Documents
Timeline and Processing Duration
| License Type | Estimated Duration | Key Milestones |
|---|---|---|
| Test License (MD13) | 1.5 – 2 months | Application, review, test license issuance |
| Manufacturing License (MD9) | 4 – 5 months (including test license) | Product testing, audit, query resolution, license grant |
| Import License (MD15) | 5 – 6 months | Document review, query resolution, license grant |
Timely preparation and proactive query management can help avoid delays.
Government Fees and Costs
The fee structure for Class C devices is as follows:
- MD9 Manufacturing License: Rs 50,000 per application + Rs 1,000 per product
- MD15 Import License:
- Rs 3,000 per site
- Rs 1,500 per product
Additional costs include testing fees at government-approved laboratories, audit fees if applicable, and consultancy fees if you seek expert support.
Common Challenges and Practical Solutions
Incomplete Documentation: Ensure all documents are current, complete, and verified. Our checklists help prevent missing paperwork.
Delayed Testing: Schedule testing well in advance with accredited labs. Confirm turnaround times to align with your submission timeline.
Audit Non-Compliance: Prepare your facility thoroughly for CDSCO audits by pre-assessing compliance and training staff.
Query Management: Respond comprehensively and promptly to any CDSCO queries to avoid prolonged delays.
Regulatory Updates: Stay informed about changes in guidelines or notifications, such as Notification 29/Misc./03/2020-DC (146) dated 26.07.2021.
Expert Consultation and Support
Navigating CDSCO licensing for a high-risk device like Nuclear Tomography Systems can be complex. Our extensive experience and dedicated support teams offer:
- Tailored regulatory strategy
- Document preparation and review
- Coordination with testing laboratories
- Audit readiness training
- Liaison with CDSCO officials
Partnering with experts accelerates approvals and ensures compliance.
Getting Started with Your CDSCO License Application
To initiate your licensing journey:
- Register on the CDSCO MD Online Portal.
- Assess your device classification and confirm applicable license type.
- Prepare detailed Device and Plant Master Files.
- Apply for the Test License (MD13) to commence product testing.
- Engage a qualified regulatory consultant or team to assist with documentation and audit preparation.
- Plan timelines aligning testing, audit, and license application carefully.
With meticulous preparation and expert guidance, you can successfully obtain your MD9 manufacturing or MD15 import license for Nuclear Tomography Systems and gain a competitive advantage in the growing Indian medical device market.