CDSCO License for Phorometer
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An ophthalmic instrument intended to be used to test ocular balance.
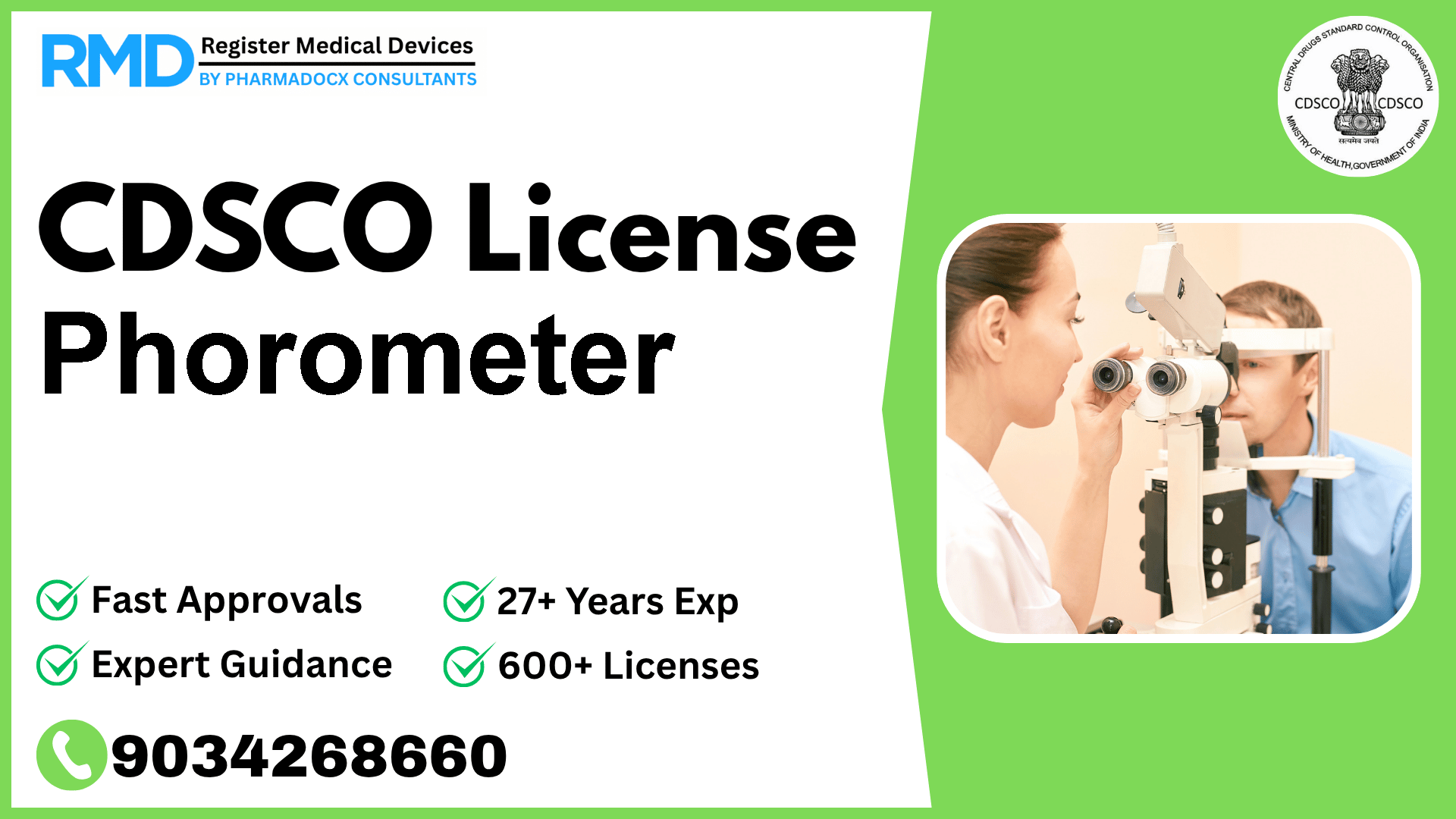
Comprehensive Guide to CDSCO Licensing for Phorometer (Class A Ophthalmic Device)
Phorometers, essential ophthalmic instruments designed to test ocular balance, play a crucial role in eye care diagnostics. For manufacturers and importers targeting the Indian market, securing a CDSCO license is a mandatory regulatory step to ensure compliance and market access. With over 25 years of experience assisting 500+ companies, we provide a detailed, practical roadmap tailored specifically for Class A devices like the Phorometer.
Understanding the CDSCO Regulatory Framework for Phorometers
Phorometers fall under the ophthalmology category and are classified as Class A medical devices according to the notification Fts No. 29/MiscJO3/2020-DC (187) dated August 9, 2021. Being a low-risk device, the regulatory oversight is managed primarily at the state level through the issuance of an MD5 license.
The Central Drugs Standard Control Organization (CDSCO) mandates that all medical devices comply with the Medical Device Rules (MDR) 2017, which define classification, licensing, testing, and audit requirements. This ensures patient safety and device efficacy.
Risk Classification and License Requirements for Phorometers
- Risk Class: A (Low Risk)
- Applicable License: MD5 Manufacturing License (Form MD3)
- Licensing Authority: State Licensing Authority
- Total Processing Time: Approximately 3-4 months
- Fees: ₹5,000 application fee + ₹500 per product
This classification means the Phorometer requires a state-level manufacturing license after successful completion of product testing and audits.
The Manufacturing License Process for Phorometer (MD5)
The path to obtaining an MD5 license for your Phorometer involves several structured steps:
Test License Acquisition (Form MD13): Before manufacturing approval, you must obtain a test license. This allows sample testing of the Phorometer in government-approved laboratories. The test license process typically takes 1.5 to 2 months.
Product Testing: The Phorometer must be tested at a CDSCO-recognized testing laboratory to verify compliance with Indian standards. You can consult the list of testing laboratories authorized for this purpose.
Documentation Preparation: Prepare comprehensive documentation including Device Master File (DMF), Plant Master File (PMF), risk management file, and quality management system (QMS) records.
Application Submission: File your MD5 application via the CDSCO MD Online Portal using Form MD3.
Audit by Notified Body: An audit by a notified body listed on the CDSCO portal assesses your manufacturing facility’s compliance. You can check the notified bodies list to select an auditor.
Query Resolution: Respond promptly to any queries raised by the CDSCO or notified body.
License Grant: Upon satisfactory evaluation, the MD5 license is granted, enabling legal manufacturing and sale of the Phorometer in India.
Manufacturing License Documents Required
To expedite the application, ensure you have the following documents ready:
- Company Constitution (Certificate of Incorporation or Partnership Deed)
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and qualifications of Technical Staff
- Fire Safety NOC
- Pollution Control Board NOC
- Device Master File (DMF) detailing design, specifications, and manufacturing processes (Device Master File Guide)
- Plant Master File (PMF) describing the manufacturing facility (Plant Master File Guide)
- Essential Principles Compliance Checklist
- Risk Management File (Risk Management)
- Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documentation (ISO 13485:2016 certified preferred)
Import License Process for Phorometer (MD15)
If you are importing Phorometers rather than manufacturing locally, an MD15 import license from the Central Licensing Authority is required. The process differs:
- No test license is required.
- Submit Form MD14 application via the CDSCO MD Online Portal.
- Provide documents such as manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE certificate, DMF, PMF, wholesale license, and company constitution.
- The total timeline ranges from 5 to 6 months.
- Fee structure varies by risk class; for Class A devices, it is approximately 50 per product.
For detailed guidance, refer to our Import License Guide.
Typical Timelines and Processing Duration
| Step | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Product Testing | 2 - 3 weeks |
| Documentation Preparation | 2 - 3 weeks |
| Application Review | 1 month |
| Notified Body Audit | 2 - 3 weeks |
| Query Resolution | 2 - 4 weeks |
| Total | 3 - 4 months |
Government Fees and Costs Breakdown
- Test License (MD13): Approx. ₹5,000
- MD5 License Application (Form MD3): ₹5,000 per application
- Product-wise Fee: ₹500 per product (Phorometer)
- Audit Fees: Varies by notified body; typically ranges from ₹50,000 to ₹1,00,000
Budgeting accurately for these costs early on is crucial to avoid delays caused by incomplete payments.
Common Challenges and Practical Solutions
1. Delays in Test License Approval:
- Tip: Submit complete documentation and promptly respond to queries.
2. Testing Laboratory Backlogs:
- Tip: Choose less busy approved labs from the CDSCO testing labs list or schedule testing well in advance.
3. Audit Non-Compliance Findings:
- Tip: Conduct internal pre-audits with experienced consultants to identify gaps before the official audit.
4. Documentation Gaps:
- Tip: Use standardized templates for DMF and PMF and maintain a robust QMS; refer to our MD5 License Guide for detailed document checklists.
Expert Consultation and Support
Navigating the regulatory landscape for medical devices like the Phorometer can be complex. Our team has successfully guided over 500 companies through the CDSCO license approval process, ensuring compliance with minimal delays. We offer:
- Comprehensive audit readiness checks
- Tailored documentation preparation
- Stepwise application submission support
- Liaison with CDSCO officials and notified bodies
Engaging expert support can shorten timelines and improve your chances of a smooth license grant.
Getting Started with Your CDSCO License Application
- Assess your manufacturing site and technical team readiness.
- Begin preparation of your Device Master File and Plant Master File using proven templates.
- Apply for the test license (Form MD13) via the CDSCO MD Online Portal.
- Schedule product testing at CDSCO-approved labs early to avoid bottlenecks.
- Prepare for the notified body audit by conducting internal pre-assessments.
- Submit your MD5 license application (Form MD3) with complete documentation.
- Stay proactive in responding to departmental queries.
Starting with these practical steps will set you on a clear path to regulatory compliance and market entry in India.
For more detailed assistance, connect with our regulatory experts who specialize in ophthalmic devices and Class A medical device licensing.
By following this expert guide and leveraging our 25+ years of industry experience, your Phorometer manufacturing or import operation can achieve timely CDSCO licensing with confidence and compliance.