CDSCO License for Polatest
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
An ophthalmic device used for evaluating hidden (latent) squinting, i.e., when the patient is not aware of the condition, and also when it cannot be seen.
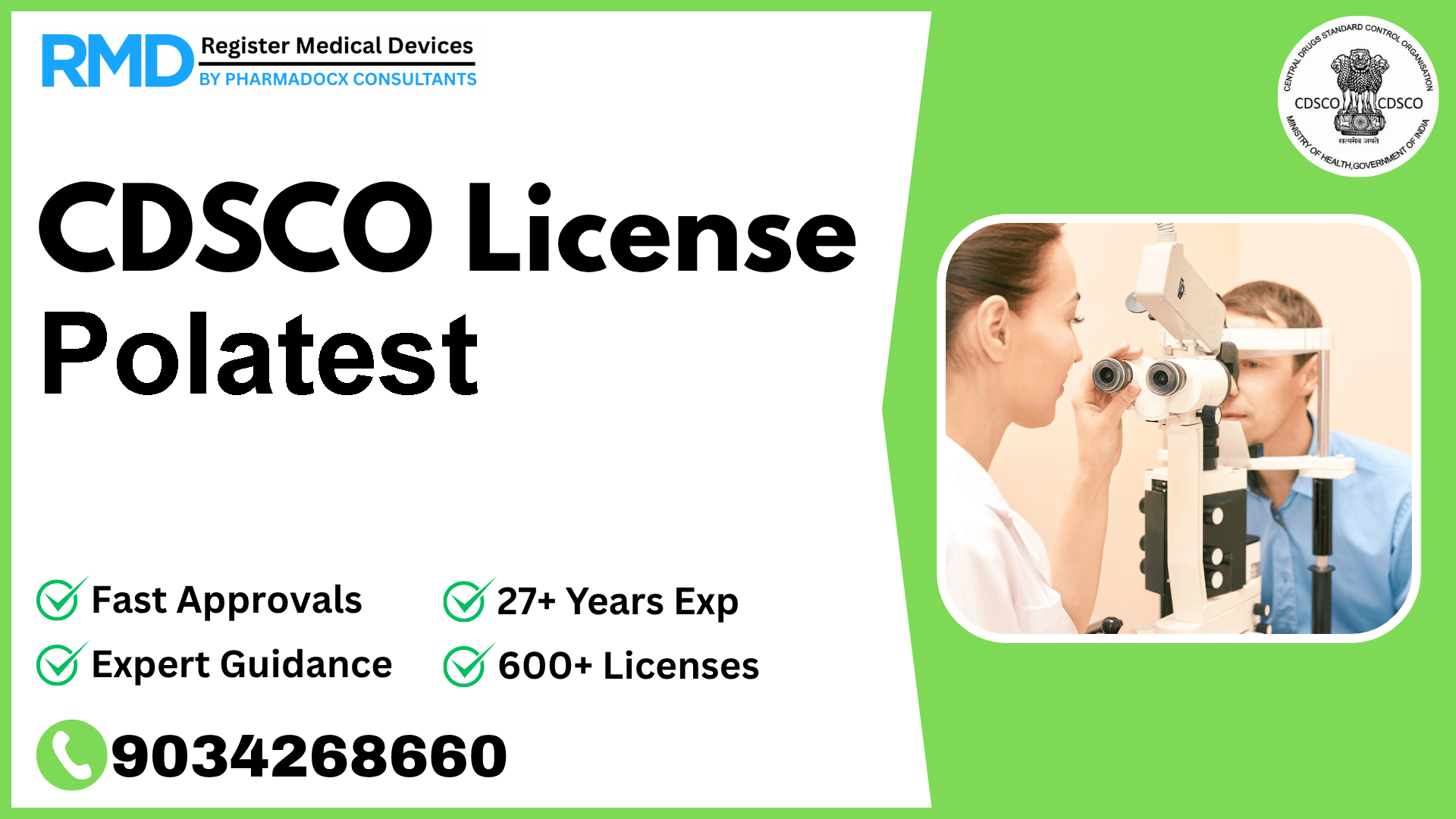
Comprehensive Guide to CDSCO Licensing for Polatest Ophthalmic Device
As seasoned regulatory consultants with over 25 years of experience and having successfully guided 500+ medical device companies through the CDSCO licensing maze, we understand the unique challenges manufacturers of Class A devices like Polatest face. Polatest, an ophthalmic device designed to detect latent squinting — a hidden eye condition not evident to patients or observers — falls under the Class A risk category as per the notification Fts No. 29/MiscJO3/2020-DC (187). This classification dictates specific regulatory pathways governed by the Central Drugs Standard Control Organization (CDSCO).
CDSCO Regulatory Framework for Polatest (Class A Ophthalmic Device)
Polatest is categorized under ophthalmology devices, intended for clinical evaluation of subtle eye misalignments. Given its low-risk classification (Class A), the regulatory framework requires a Manufacturing License (MD5) issued by the State Licensing Authority. This license ensures that manufacturers comply with quality, safety, and performance standards mandated by CDSCO.
The governance of Polatest's approval is aligned with the Medical Devices Rules, 2017, and subsequent notifications including the August 9, 2021 update that specifically mentions this device.
Risk Classification and License Requirements for Polatest
- Risk Class: A (Low risk)
- License Type: MD5 (Manufacturing License for Class A and B devices)
- Regulatory Authority: State Licensing Authority
- Application Form: MD3
- Process Duration: Approximately 3-4 months (inclusive of test license, testing, audit, and license grant)
For a deeper understanding of medical device classification, manufacturers can refer to Medical Device Classification.
Manufacturing License Process for Polatest (MD5)
Obtaining an MD5 license involves a structured multi-step process:
Test License on Form MD13: Before full manufacturing license approval, manufacturers must obtain a test license for Polatest, allowing limited production for product testing. This step usually takes 1.5 to 2 months.
Product Testing: Polatest must be tested at CDSCO-approved government laboratories to verify compliance with safety and performance standards. Refer to the Testing Laboratories List for authorized labs.
Documentation Preparation: This involves compiling detailed technical and regulatory documents including Device Master File, Plant Master File, and risk assessment files.
Application Submission: Submit the licensing application via the CDSCO MD Online Portal using Form MD3.
Audit by Notified Body: A notified body conducts an on-site audit of manufacturing premises and quality systems. Manufacturers can check the Notified Bodies List to select an appropriate auditor.
Query Resolution: Address any questions or clarifications raised by the CDSCO or notified body promptly.
License Grant: Upon successful audit and document verification, the MD5 license is granted, allowing full-scale manufacturing.
For a detailed walkthrough, check our MD5 License Guide.
Manufacturing License Documents Required for Polatest
Manufacturers must prepare and submit comprehensive documentation, including but not limited to:
- Company constitution and incorporation certificates
- Proof of ownership or lease of manufacturing premises
- Qualification and experience documents of technical staff
- Fire safety and pollution control clearances (NOCs)
- Device Master File (DMF): Details design, specifications, and manufacturing processes (Device Master File Guide)
- Plant Master File (PMF): Details of the manufacturing facility and quality systems (Plant Master File Guide)
- Essential Principles Compliance Checklist
- Risk Management File highlighting hazard analyses and mitigations (Risk Management)
- Test reports from CDSCO-approved laboratories
- Labels, Instructions for Use (IFU), and packaging details
- Quality Management System (QMS) documentation, typically ISO 13485:2016 compliant
Import License Process for Polatest (MD15)
While Polatest’s manufacturing license is primarily handled at the state level (MD5), importers looking to bring Polatest into India require an MD15 Import License issued by the Central Licensing Authority.
The import license process includes:
- Comprehensive document preparation
- Application submission via the CDSCO MD Online Portal
- Resolution of departmental queries
- Final license issuance
Detailed guidance is available in our Import License Guide.
Import License Documents Required for Polatest
Importers must furnish:
- Valid manufacturing license (MD5 or MD9) from the country of origin
- Free Sale Certificate from the country of origin
- ISO 13485:2016 certificate
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale license
- Company constitution documents
Timeline and Processing Duration
| License Type | Steps Included | Approximate Duration |
|---|---|---|
| Test License (MD13) | Application, testing, approval | 1.5 - 2 months |
| Manufacturing License (MD5) | Document preparation, audit, queries, license grant | 3 - 4 months (including test license) |
| Import License (MD15) | Document submission, query resolution, grant | 5 - 6 months |
By planning ahead and ensuring thorough documentation, manufacturers can minimize delays.
Government Fees and Costs for Polatest Licensing
MD5 Manufacturing License:
- Application fee: INR 5,000
- Per product fee: INR 500 (applies to Polatest as one product)
Test License (MD13): Typically included within the MD5 process
Import License (MD15): Fees vary by device class; for Class A devices, approximately 50 per product
These fees are payable via the CDSCO MD Online Portal during application submission.
Common Challenges and Solutions
Challenge: Delays in obtaining test license or laboratory analysis
- Solution: Engage early with government-approved labs and submit test samples promptly; track progress actively.
Challenge: Incomplete or inconsistent document submission
- Solution: Use checklists and templates from experienced consultants; ensure all technical and quality documents are aligned.
Challenge: Audit non-conformities
- Solution: Pre-audit internal reviews and gap assessments; engage notified bodies for advisory audits.
Challenge: Query resolution delays
- Solution: Prepare detailed, clear responses; maintain open communication with CDSCO officials.
Expert Consultation and Support
Navigating CDSCO licensing for Polatest can be complex. Our extensive experience with over 500 successful client engagements means we provide tailored support, from document preparation to audit facilitation and post-license compliance. We help manufacturers streamline the process, avoid common pitfalls, and achieve faster time-to-market.
Getting Started with Your CDSCO License Application for Polatest
- Assess your classification: Confirm Polatest’s Class A status and regulatory pathway.
- Prepare initial documentation: Start compiling company, technical, and quality documents, focusing on Device and Plant Master Files.
- Apply for Test License (MD13): Submit your test license application on the CDSCO MD Online Portal.
- Coordinate product testing: Engage a CDSCO-approved lab early and submit Polatest for testing.
- Plan for audit: Select a notified body from the official list and prepare your facility and QMS for inspection.
- Submit MD5 license application (Form MD3): Once testing is complete, apply for the manufacturing license via the portal.
- Respond promptly to queries: Maintain active communication with CDSCO during the review phase.
By following these practical steps and leveraging expert guidance, manufacturers and importers can successfully navigate the CDSCO licensing process for Polatest and establish a compliant presence in the Indian ophthalmic device market.