CDSCO License for Powered patient table for accelerator
Medical Device Information
Intended Use
A bed operates by programmable for electric radiotherapy designed to adjust the patient's posture and immobilize the patient for radiotherapy that uses medical linear accelerator or non-linear accelerator.
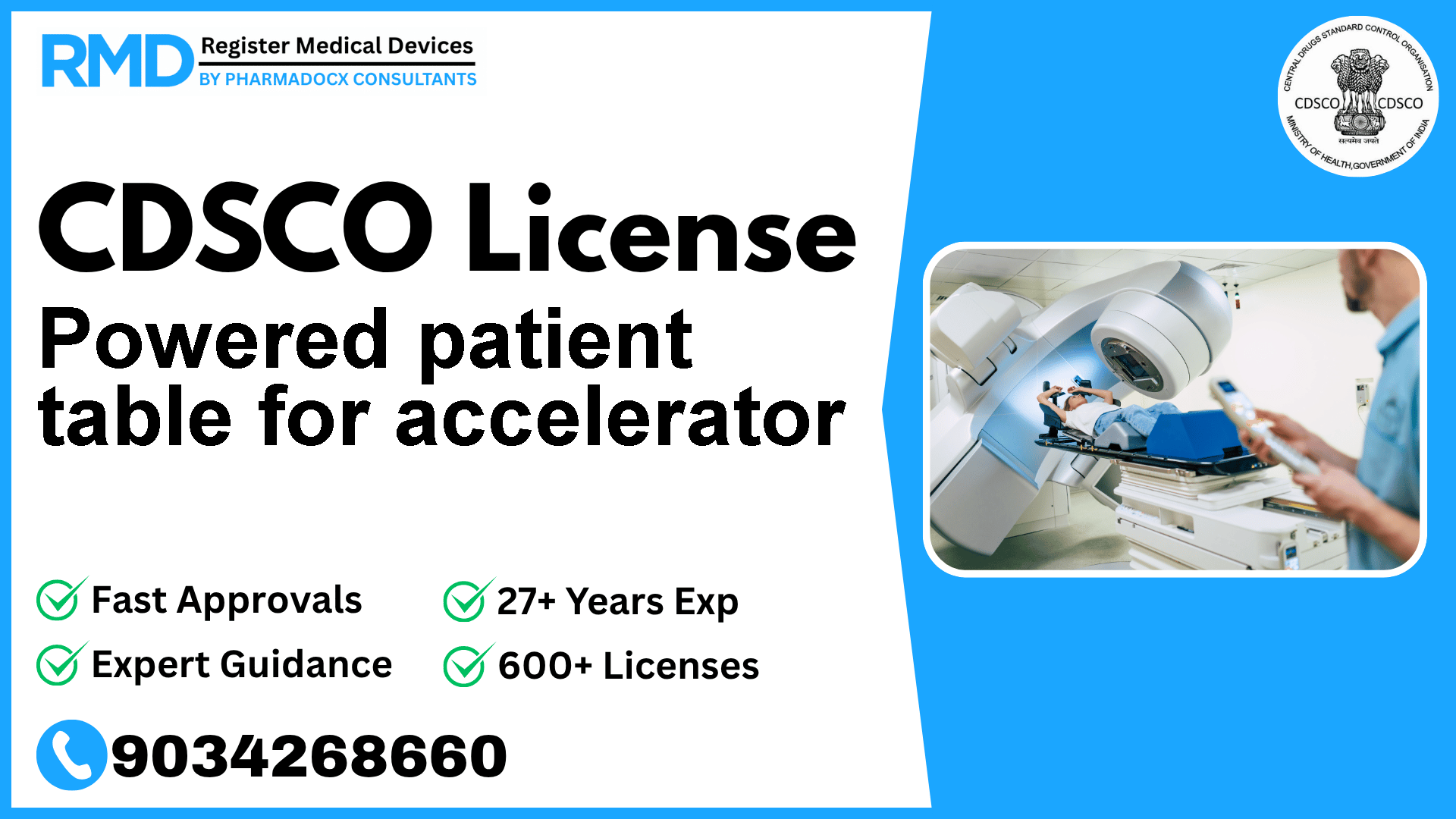
Comprehensive Guide to CDSCO Licensing for Powered Patient Table for Accelerator (Class B)
As seasoned regulatory consultants with over 25 years of experience and having supported 500+ companies in securing CDSCO licenses, we understand the nuances of medical device regulations in India. Today, we focus on the Powered Patient Table for Accelerator—a specialized Class B device used in radiotherapy to precisely position and immobilize patients during treatment with medical linear or non-linear accelerators.
Understanding the Device and Its Regulatory Importance
The Powered Patient Table for Accelerator is an electrically programmable bed designed to optimize the accuracy of radiotherapy procedures. Given its critical role in patient safety and treatment efficacy, this device falls under Class B risk category per CDSCO's classification.
Proper regulatory compliance ensures not only patient safety but also smooth market entry, avoiding costly delays or product recalls. The device is notified under File No. 29/Misc./03/2020-DC (180), dated 6.8.2021, making it subject to specific licensing requirements.
CDSCO Regulatory Framework for Powered Patient Table for Accelerator
As a Class B medical device, the Powered Patient Table requires a manufacturing license under the MD5 category, which is granted by the State Licensing Authority. Importers seeking to bring this device into India must apply for an MD15 import license from the Central Licensing Authority.
The regulatory framework involves:
- Obtaining a Test License (Form MD13) before full manufacturing license application
- Product testing at CDSCO-approved laboratories
- Submission of a comprehensive dossier including Device Master File and Plant Master File
- An audit by a Notified Body
Risk Classification and License Requirements for Class B Devices
Class B devices present moderate risk and require strict adherence to quality management systems aligned with ISO 13485 standards.
- Manufacturing License: MD5 (Application Form MD3) issued by State Licensing Authority
- Test License: MD13 (mandatory before MD5)
- Audit: Conducted by a CDSCO notified body (check the list of notified bodies)
- Testing: Product samples must be tested at government-approved labs (see Testing Laboratories)
Manufacturing License Process (MD5) for Powered Patient Table
The entire MD5 licensing process typically spans 3 to 4 months and involves the following steps:
- Apply for Test License (Form MD13): Takes approximately 1.5 to 2 months.
- Product Testing: Conduct tests at CDSCO-approved labs to verify compliance with essential principles.
- Prepare Dossier: Compile all required documents including Device Master File, Plant Master File, Risk Management File, and QMS documents.
- Submit Application for MD5 License (Form MD3): Via the CDSCO MD Online Portal.
- Audit by Notified Body: To verify manufacturing facility and quality systems.
- Respond to Queries: Address any clarifications raised by CDSCO or the notified body.
- Grant of License: Issuance of MD5 License (Form MD5).
Manufacturing License Documents Required for Class B Devices
Your dossier must include:
- Company Constitution/Registration Proof
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualification and Experience Details
- Fire Safety NOC and Pollution Control NOC
- Device Master File detailing design, specifications, and labeling (Device Master File Guide)
- Plant Master File describing manufacturing processes and quality systems (Plant Master File Guide)
- Essential Principles Checklist demonstrating compliance with Indian regulations
- Risk Management File aligned with ISO 14971 (Risk Management)
- Product Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System Documentation (ISO 13485:2016 compliance)
Import License Process (MD15) for Powered Patient Table
If you are an importer, the MD15 license is mandatory and issued by the Central Licensing Authority. The process generally takes 5 to 6 months and involves:
- Document Preparation: Including Manufacturing License of the foreign manufacturer, Free Sale Certificate, ISO 13485:2016 certificate, CE Certificate, Device and Plant Master Files, Wholesale License, and Company Constitution.
- Application Submission: Use Form MD14 via the CDSCO MD Online Portal.
- Query Resolution: Address any queries from CDSCO.
- License Grant: Issuance of MD15 Import License.
Import License Documents Required
- Valid Manufacturing License (MD5/MD9) from the manufacturer’s country
- Free Sale Certificate from the country of origin
- ISO 13485:2016 Certification
- CE Certificate or equivalent
- Device Master File and Plant Master File
- Wholesale License for Indian importer
- Company Constitution and address proof
Timeline and Processing Duration
| License Type | Processing Time |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Manufacturing (MD5) | 3 to 4 months (including audit and queries) |
| Import License (MD15) | 5 to 6 months |
Government Fees and Costs
- MD5 License: Rs 5000 per application + Rs 500 per product
- Test License (MD13): Nominal fees apply; confirm on portal
- Import License (MD15):
- Class B devices: Rs 2000 per site + Rs 1000 per product
All payments are made via the CDSCO MD Online Portal.
Common Challenges and Practical Solutions
1. Delays in Test License Approval:
- Ensure complete and accurate submission of the test license application.
- Follow up regularly on the online portal.
2. Product Testing Failures:
- Conduct pre-submission internal testing.
- Choose accredited labs listed on the CDSCO site.
3. Audit Non-Compliance:
- Maintain robust QMS as per ISO 13485.
- Prepare thoroughly with all required documents and records.
4. Query Resolution Delays:
- Respond promptly with detailed, evidence-backed clarifications.
Expert Consultation and Support
Navigating CDSCO licensing can be complex, especially for specialized devices like the Powered Patient Table for Accelerator. Our team has successfully guided over 500 manufacturers and importers through the entire lifecycle—from dossier preparation to audit and license grant.
We offer tailored services including:
- Gap analysis of your current compliance
- Documentation and dossier preparation
- Coordination of product testing with authorized labs
- Audit preparation and mock inspections
- Liaison with CDSCO and notified bodies
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm Class B status and corresponding license type (MD5).
- Register on the CDSCO MD Online Portal: This is mandatory for all submissions.
- Apply for Test License (Form MD13): This is your first step towards manufacturing license.
- Plan Product Testing: Identify and coordinate with CDSCO-approved labs.
- Prepare Documentation: Use our comprehensive guides on Device and Plant Master Files.
- Schedule Audit with a Notified Body: Choose from the list of notified bodies well in advance.
- Submit MD5 License Application (Form MD3): Once test reports and audit are complete.
For importers, start by assembling the required foreign certifications and Indian wholesale license before applying for MD15.
Ready to make your medical device compliant and market-ready? Contact us today for a personalized roadmap and expert assistance in securing your CDSCO license efficiently and without hassle.
For more detailed insights, explore our dedicated guides:
Your journey to compliant, safe, and market-ready medical devices starts here.