CDSCO License for Radiographic (Non Vascular) Catheter
Medical Device Information
Intended Use
Interventional radiologists obtain images using needles and narrow tubes called catheters, rather than by making large incisions into the body as in traditional surgery.
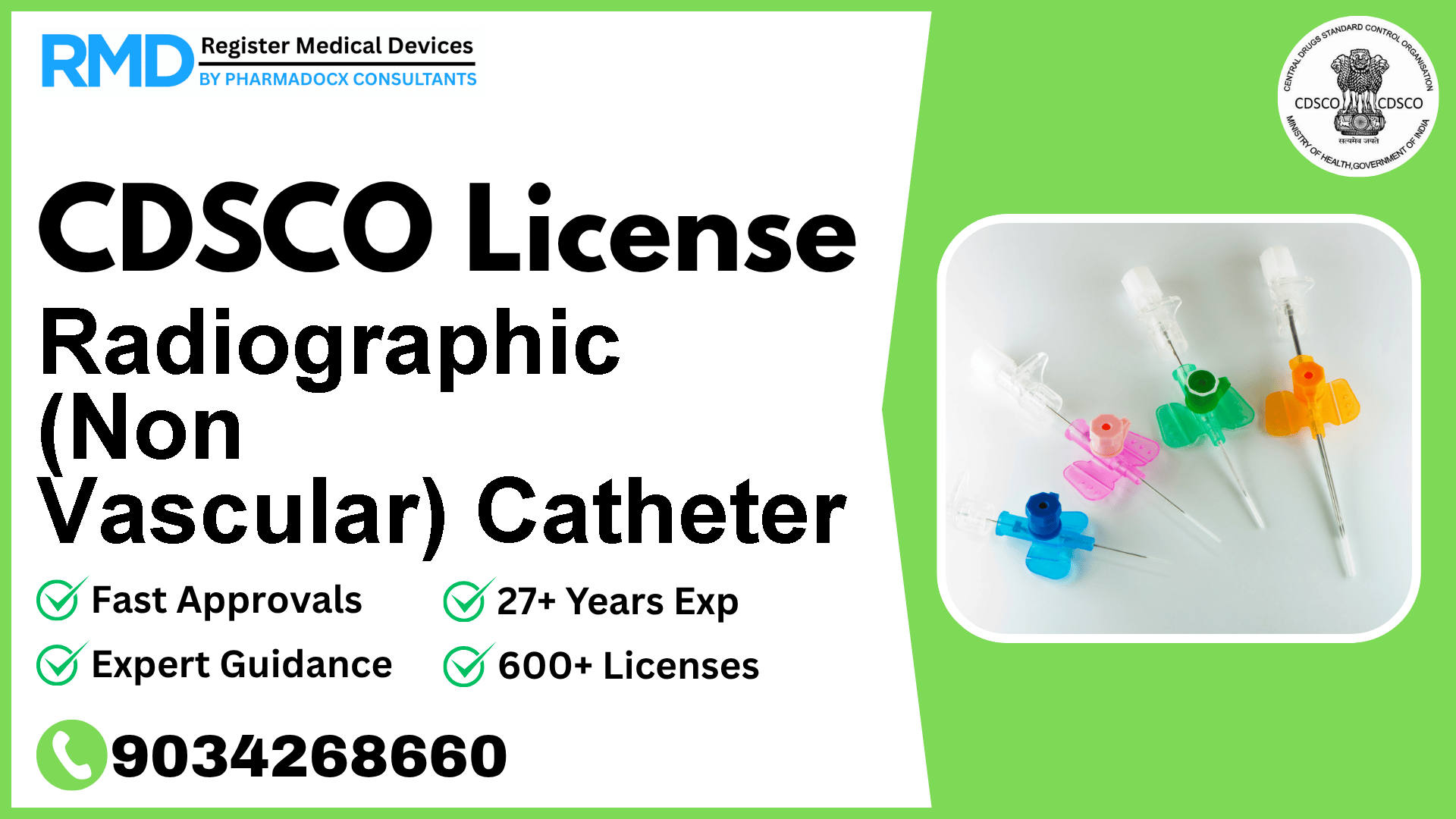
Comprehensive Guide to CDSCO Licensing for Radiographic (Non Vascular) Catheters (Risk Class B)
Radiographic (Non Vascular) Catheters are specialized medical devices used by interventional radiologists to obtain high-precision images without invasive surgery. Their minimally invasive nature requires stringent regulatory compliance to ensure patient safety. As seasoned regulatory consultants with over 25 years of experience and having supported 500+ companies, we provide detailed, actionable guidance to help manufacturers and importers secure the mandatory CDSCO license efficiently.
CDSCO Regulatory Framework for Radiographic (Non Vascular) Catheters
The Central Drugs Standard Control Organization (CDSCO) regulates medical devices in India under the Medical Device Rules 2017. Radiographic (Non Vascular) Catheters fall under the category of "Catheters" and are classified as Class B devices, indicating a low to moderate risk level.
The governing notification is 29/Misc/3/2017-DC (292), dated 06.06.2018, which mandates licensing for manufacturing and importing such devices.
Risk Classification and License Requirements for Class B Devices
Class B devices like Radiographic Catheters require obtaining an MD5 manufacturing license issued by the State Licensing Authority. This is a critical step to legally manufacture and market these devices in India. The license process involves rigorous compliance checks, including test licenses, product testing, and audits by notified bodies.
For importers, an MD15 import license issued by the Central Licensing Authority is necessary to bring these devices into India.
Manufacturing License Process (MD5) for Radiographic Catheters
The manufacturing license process for Class B Medical Devices is comprehensive yet streamlined when approached correctly:
- Test License Application (Form MD13)
- Duration: 1.5 to 2 months
- Purpose: Allows the manufacturer to conduct product testing and initial production under supervision.
- Product Testing
- Conducted at government-approved testing laboratories.
- Testing parameters align with the Essential Principles Checklist and risk management guidelines.
- Document Preparation & Submission (Form MD3)
- Submission through the CDSCO MD Online Portal.
- Audit by Notified Body
- Conducted by bodies listed in the Notified Bodies List for MD5 Audit.
- Resolution of Queries
- Addressing any observations from CDSCO or notified body.
- Grant of License (Form MD5)
Manufacturing License Documents Required for Radiographic Catheters
Accuracy and completeness in documentation expedite the licensing process. Essential documents include:
- Company Constitution (Incorporation Certificate, Memorandum & Articles of Association)
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Qualification and Experience Details of Technical Staff
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) – detailed product specifications and manufacturing process (Device Master File Guide)
- Plant Master File (PMF) – facility details and quality control systems (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with safety and performance requirements
- Risk Management File documenting hazard analysis and mitigation strategies (Risk Management)
- Test Reports from government-approved laboratories (Testing Laboratories)
- Device Labels and Instructions for Use (IFU)
- Quality Management System (QMS) Documents (typically ISO 13485:2016 compliant)
Import License Process (MD15) for Radiographic Catheters
Importers of Radiographic Catheters must obtain an MD15 import license from CDSCO’s Central Licensing Authority. The process includes:
- Preparation of required documents including Manufacturing License from country of origin, Free Sale Certificate, ISO 13485:2016 certificate, CE Certificate, DMF, PMF, Wholesale License, and Company Constitution.
- Application submission using Form MD14 on the CDSCO MD Online Portal.
- Resolution of any queries raised by CDSCO during the review.
- Grant of MD15 license enabling import and sale in India.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate (FSC)
- ISO 13485:2016 Certification
- CE Certificate (if applicable)
- Device Master File
- Plant Master File
- Wholesale License
- Company Constitution
Timeline and Processing Duration
| License Type | Duration |
|---|---|
| Test License (MD13) | 1.5 - 2 months |
| Manufacturing License (MD5) | 3 - 4 months (including test license, testing, audit) |
| Import License (MD15) | 5 - 6 months |
Government Fees and Costs
For Class B Radiographic Catheters, the fee structure is as follows:
- MD5 Manufacturing License:
- Rs 5,000 per application
- Rs 500 per product
- MD15 Import License:
- $2,000 per site
- $1,000 per product
Note: Additional costs may include testing fees at government-approved laboratories and audit fees charged by notified bodies.
Common Challenges and Solutions
Challenge 1: Delays in Test License Approval
- Solution: Prepare and submit comprehensive, error-free documentation from the outset. Early engagement with notified bodies can preempt issues.
Challenge 2: Incomplete Risk Management Documentation
- Solution: Implement a robust risk management system aligned with ISO 14971 principles and maintain thorough records.
Challenge 3: Non-compliance during Audit
- Solution: Conduct internal audits and mock inspections before the official audit. Use checklists aligned with CDSCO expectations.
Challenge 4: Product Testing Failures
- Solution: Partner with qualified testing laboratories early to understand test requirements and ensure product readiness.
Expert Consultation and Support
Navigating CDSCO licensing for Radiographic Catheters can be complex. Our consultancy leverages 25+ years of regulatory expertise to:
- Conduct gap assessments and readiness audits
- Prepare and review Device and Plant Master Files
- Liaise with CDSCO and notified bodies
- Facilitate product testing and documentation
- Expedite query resolution and license grant
Getting Started with Your CDSCO License Application
- Identify Your Risk Class and Applicable License — Confirm your device is Class B and requires an MD5 license.
- Register on the CDSCO MD Online Portal — Begin your application process by registering here: CDSCO MD Online Portal.
- Prepare Test License Application (Form MD13) — Gather documentation and submit to obtain permission for product testing.
- Arrange Product Testing — Coordinate with government-approved laboratories to conduct necessary tests.
- Compile Comprehensive Documentation — Assemble all required files including DMF, PMF, risk management, and QMS documents.
- Schedule Audit with Notified Body — Refer to the list of notified bodies and arrange audits.
- Submit Manufacturing License Application (Form MD3) — Complete the formal submission after test license and audit.
- Prepare for Queries — Respond promptly and accurately to any CDSCO or notified body queries.
By following these steps with expert guidance, manufacturers and importers of Radiographic (Non Vascular) Catheters can successfully navigate CDSCO licensing requirements and enter the Indian market with confidence.
For personalized assistance, compliance consulting, and turnkey solutions, contact us today to leverage our proven track record in CDSCO licensing success.