CDSCO License for Radionuclide radiation therapy system.
Medical Device Information
Intended Use
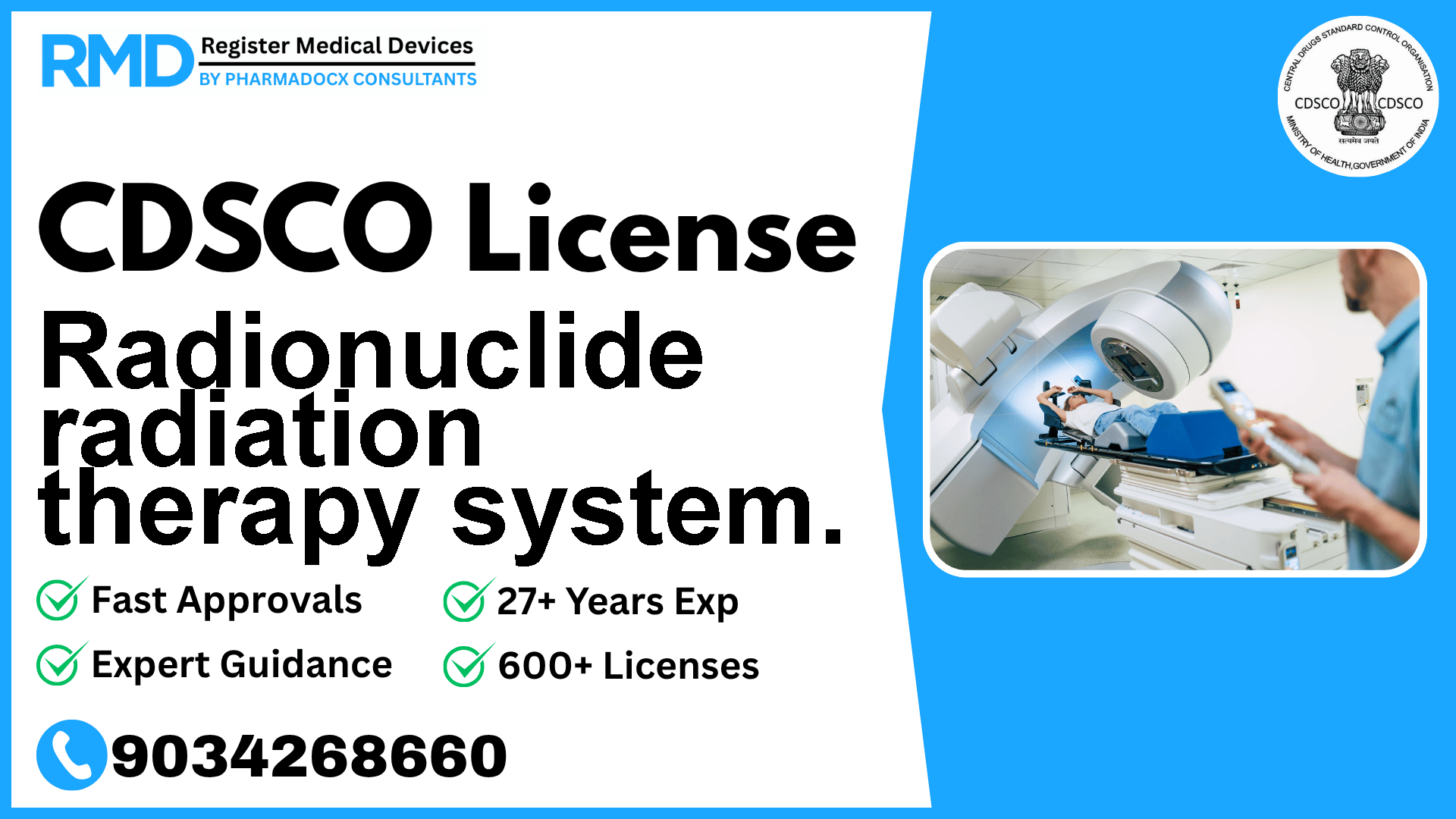
A radionuclide radiation therapy system is a device intended to permit an operator to administer gamma radiation therapy, with the radiation source located at a distance from the patient's body.
Comprehensive Guide to CDSCO Licensing for Radionuclide Radiation Therapy Systems (Class C Medical Device)
Radionuclide radiation therapy systems are vital medical devices designed to administer gamma radiation therapy remotely, ensuring precision in cancer treatment. Given their complexity and potential risks, these devices fall under Class C according to the CDSCO classification, necessitating stringent regulatory compliance. As seasoned regulatory consultants with over 25 years of experience and having supported 500+ companies, we understand the nuances in obtaining CDSCO licenses for such advanced radiotherapy equipment. This guide offers you detailed, actionable insights into the regulatory process, documentation, timelines, and costs specific to your device.
CDSCO Regulatory Framework for Radionuclide Radiation Therapy Systems
The Central Drugs Standard Control Organization (CDSCO) governs the import and manufacture of medical devices in India under the Medical Device Rules (MDR) 2017. Since your device falls under the radiotherapy category and is classified as Class C, it comes under the purview of the Central Licensing Authority.
Your device notification details:
- File No.: 29/Misc./03/2020-DC (180)
- Notification Date: 6.8.2021
This notification confirms your device is recognized and regulated under the current framework, ensuring clarity on compliance requirements.
Risk Classification and License Requirements for Class C Devices
Class C devices, including radionuclide radiation therapy systems, are considered moderate to high risk. Consequently, they require an MD9 manufacturing license granted by the CDSCO Central Licensing Authority.
Key points:
- License Type: MD9 (Application Form MD7)
- Regulatory Authority: Central Licensing Authority
- Typical Processing Time: 4 to 5 months
- Fees: Rs. 50,000 per application + Rs. 1,000 per product
For importers, the relevant license is the MD15 import license, also issued by the Central Authority.
Manufacturing License Process for Radionuclide Radiation Therapy Systems (MD9 License)
Obtaining an MD9 license is a multi-step process, structured to ensure safety and efficacy:
Test License Application (Form MD13): Before the MD9 license, a test license must be secured, which takes approximately 1.5 to 2 months. This allows you to test your device legally.
Product Testing: Devices must undergo testing at CDSCO-approved laboratories to confirm compliance with applicable standards. You can find the list of testing laboratories here.
Document Preparation: Comprehensive documentation, including Device Master File, Plant Master File, and Risk Management File, must be meticulously prepared.
Application Submission: Submit the MD9 application through the CDSCO MD Online Portal.
Audit and Inspection: CDSCO inspectors conduct an audit of your manufacturing facility and quality management system.
Query Resolution: Respond promptly to any queries raised by the CDSCO during review.
License Grant: On successful review and audit, the MD9 license is granted.
For a detailed stepwise guide, our MD9 License Guide provides valuable insights.
Manufacturing License Documents Required
To streamline your application, ensure you have the following documentation ready:
- Company Constitution (Registration Certificate)
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualifications of Technical Staff (including biomedical engineers, quality managers)
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design, specifications, and production process (See our DMF guide)
- Plant Master File (PMF) describing manufacturing facilities and environment (PMF guide here)
- Essential Principles Checklist confirming compliance with Indian Medical Device Rules
- Risk Management File demonstrating hazard identification and mitigation (Risk management insights)
- Test Reports from CDSCO-approved labs
- Labels and Instructions for Use (IFU) as per regulatory standards
- Quality Management System Documents (ISO 13485:2016 certification is highly recommended)
Having these documents well-prepared can significantly reduce review time.
Import License Process for Radionuclide Radiation Therapy Systems (MD15 License)
Manufacturers or importers intending to bring the device into India must obtain an MD15 import license:
- Application Form: MD14
- Authority: Central Licensing Authority
- Typical Timeline: 5 to 6 months
- No Test License Required
The process involves thorough document evaluation and verification, followed by a license grant.
Import License Documents Required
Key documents for MD15 include:
- Valid Manufacturing License (MD9) from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certification
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License
- Company Constitution
Submission is done via the CDSCO MD Online Portal.
Timeline and Processing Duration
| License Type | Typical Duration | Steps Involved |
|---|---|---|
| MD13 Test License | 1.5 - 2 months | Application, review, grant |
| MD9 Manufacturing License | 4 - 5 months | Test license, testing, audit, queries, grant |
| MD15 Import License | 5 - 6 months | Document review, queries, grant |
Planning ahead and adhering to timelines can prevent unnecessary delays.
Government Fees and Costs
- MD9 License: Rs. 50,000 per application + Rs. 1,000 per product
- MD13 Test License: Nominal fees (varies by state)
- MD15 Import License: For Class C devices, approx Rs. 3,000 per site + Rs. 1,500 per product
Budgeting for these fees early is crucial in your project plan.
Common Challenges and Solutions
Challenge: Delays in testing due to limited slots at approved labs.
Solution: Schedule your testing early and consider multiple labs from the CDSCO-approved list.
Challenge: Incomplete or inconsistent documentation leads to repeated queries.
Solution: Employ professional regulatory consultants to review your Device Master File and Risk Management File before submission.
Challenge: Audit non-compliance due to inadequate QMS.
Solution: Implement ISO 13485:2016 compliant Quality Management Systems and conduct internal audits prior to CDSCO inspection.
Expert Consultation and Support
Navigating CDSCO licensing for Class C devices like radionuclide radiation therapy systems is complex. Our expertise, backed by over 25 years and 500+ successful licensing projects, ensures you avoid pitfalls and accelerate approval. From documentation to audit readiness, our tailored support covers every aspect.
Getting Started with Your CDSCO License Application
- Assess Device Classification: Confirm your device classification and applicable license type.
- Prepare Test License Application: Begin with the MD13 application to secure legal testing approval.
- Engage Accredited Testing Labs: Book testing slots early to avoid delays.
- Compile Documentation: Use our Device Master File and Plant Master File guides for thorough preparation.
- Submit Applications Online: Use the official CDSCO MD Online Portal for all submissions.
- Prepare for Audits: Coordinate with notified bodies or CDSCO inspectors for smooth audits; consult the list of notified bodies.
- Respond Promptly to Queries: Maintain clear communication channels with CDSCO.
By following these steps and leveraging expert support, you can confidently enter the Indian radiotherapy device market with compliance and efficiency.
For personalized assistance with your Radionuclide Radiation Therapy System licensing, contact us to leverage our extensive CDSCO expertise and ensure a hassle-free journey to market authorization.