CDSCO License for Retention Type Balloon Catheter
Medical Device Information
Intended Use
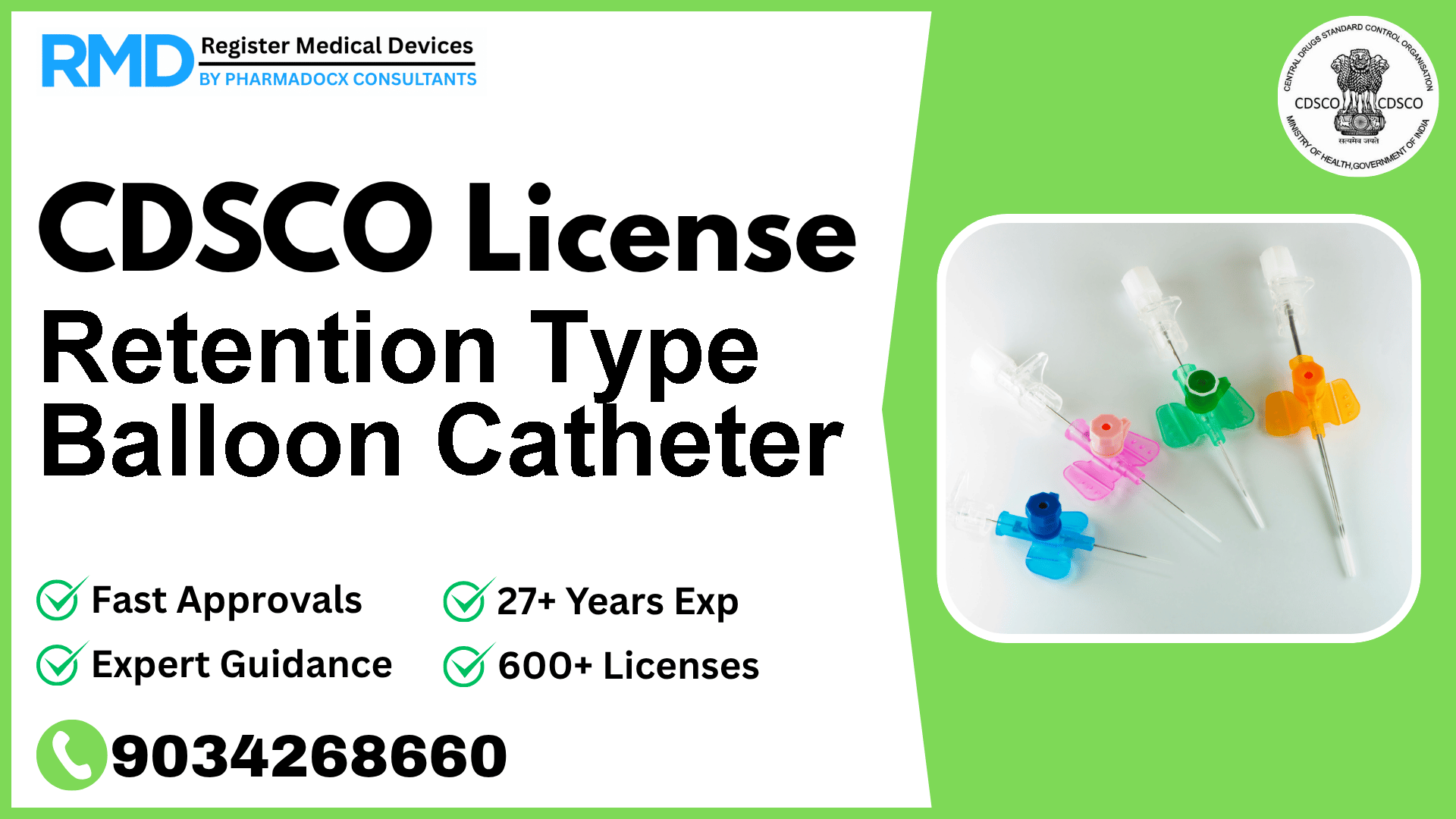
It has a balloon at the distal end, which is inflated with sterile water or saline to
Comprehensive Guide to CDSCO Licensing for Retention Type Balloon Catheters (Class B)
As a medical device regulatory consultancy with over 25 years of experience and a track record of assisting over 500 companies, we understand the intricacies involved in obtaining CDSCO licenses for devices like the Retention Type Balloon Catheter. This device, categorized under catheters and classified as Class B risk, requires adherence to specific regulatory pathways to ensure smooth market entry in India.
Introduction: Retention Type Balloon Catheter and Regulatory Importance
Retention Type Balloon Catheters are specialized medical devices featuring an inflatable balloon at the distal end, typically filled with sterile water or saline. This design allows the catheter to remain securely in place during medical procedures. Given their direct interface with body fluids and tissues, regulatory oversight ensures patient safety and product efficacy.
In India, the Central Drugs Standard Control Organization (CDSCO) governs the licensing and regulation of such devices. Compliance with CDSCO regulations is mandatory before manufacturing or importing this device into the Indian market.
CDSCO Regulatory Framework for Retention Type Balloon Catheters
Retention Type Balloon Catheters fall under Class B medical devices according to notified classification Notification No. 29/Misc/3/2017-DC (292) dated 06.06.2018. Class B devices are considered low to moderate risk and require a manufacturing license (MD5) issued by the State Licensing Authority.
The licensing process involves obtaining a test license (MD13), product testing from CDSCO-approved labs, document submission, and an audit by a notified body.
Risk Classification and License Requirements
| Device Name | Risk Class | License Type | Licensing Authority | Timeline | Fees (INR) |
|---|---|---|---|---|---|
| Retention Type Balloon Catheter | B | MD5 | State Licensing Authority | 3-4 months (total) | Application: Rs 5,000 + Rs 500 per product |
For Class B devices, the MD5 license is essential for manufacturing, while importers require an MD15 license from CDSCO Central Licensing Authority.
Manufacturing License Process (MD5) for Retention Type Balloon Catheters
The MD5 license process for Class B devices includes the following steps:
Test License Application (Form MD13): Submit a test license application to the State Licensing Authority. The test license permits manufacturing and testing of samples. Processing time is approximately 1.5-2 months.
Product Testing: Coordinate with CDSCO-approved testing laboratories to perform the mandatory product tests. Refer to the list of testing laboratories.
Document Preparation: Compile all necessary documentation, including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, test reports, labels, and Instructions for Use (IFU).
License Application (Form MD3): Apply for the MD5 license through the CDSCO MD Online Portal.
Audit by Notified Body: Arrange for an audit by a notified body as per the Notified Bodies List. The audit evaluates compliance with Good Manufacturing Practices and QMS requirements.
Query Resolution: Address any queries or observations raised by the licensing authority or notified body.
Grant of License (Form MD5): Upon satisfactory compliance, the State Licensing Authority issues the manufacturing license.
Manufacturing License Documents Required
To ensure a smooth application process, the following documents must be submitted:
- Company Constitution (Incorporation Certificate, MoA, AoA)
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Documents of Technical Personnel
- Fire and Pollution NOCs
- Device Master File (DMF) detailing design, manufacturing process, and specifications (Device Master File Guide)
- Plant Master File describing manufacturing facilities and equipment (Plant Master File Guide)
- Essential Principles Checklist for compliance with Indian Medical Device Rules
- Risk Management File demonstrating hazard identification and mitigation (Risk Management)
- Test Reports from CDSCO-approved laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, typically ISO 13485:2016 certification
Import License Process (MD15) for Retention Type Balloon Catheters
If you are an importer of Retention Type Balloon Catheters, the MD15 license from CDSCO Central Licensing Authority is mandatory. The process includes:
Document Compilation: Gather required documents, including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate (if applicable), Device and Plant Master Files, Wholesale License, and Company Constitution.
Application Submission (Form MD14): Apply online via the CDSCO MD Online Portal with all supporting documents.
Query Resolution: Address any clarifications requested by CDSCO.
Grant of License (Form MD15): After successful evaluation, the import license is granted, typically within 5-6 months.
Import License Documents Required
- Valid Manufacturing License of the product in the country of origin
- Free Sale Certificate issued by the competent authority of the exporting country
- ISO 13485:2016 Certificate
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License for distribution in India
- Company Constitution documents
Timeline and Processing Duration
| Process Step | Approximate Duration |
|---|---|
| Test License (MD13) Processing | 1.5 - 2 months |
| Product Testing | 2 - 4 weeks |
| Document Preparation | 2 - 3 weeks |
| MD5 License Application & Audit | 1 - 1.5 months |
| Query Resolution | 2 - 4 weeks |
| Total for MD5 License | 3 - 4 months |
For import licenses (MD15), expect 5-6 months from application to grant.
Government Fees and Costs
- MD5 License: Application fee Rs 5,000 + Rs 500 per product
- MD13 Test License: Included in MD5 process fees
- MD15 Import License:
- Class B devices: Rs 2,000 per site + Rs 1,000 per product
Additional costs may include notified body audit fees, product testing charges, and consultancy fees if you engage expert support.
Common Challenges and Solutions
Challenge: Delays in product testing due to limited slots at government-approved labs.
Solution: Early coordination with testing labs and advance scheduling can mitigate delays. Utilize the official Testing Laboratories list to select labs with shorter lead times.
Challenge: Incomplete documentation leading to queries.
Solution: Comprehensive preparation using checklists and expert review ensures all technical files and QMS documents meet CDSCO requirements.
Challenge: Audit non-conformities.
Solution: Conduct internal audits and gap assessments before notified body visits to preempt compliance issues.
Expert Consultation and Support
Navigating CDSCO licensing can be complex. Our experienced team offers tailored consultancy for Retention Type Balloon Catheters, including:
- Gap analysis and documentation support
- Coordination with testing labs and notified bodies
- Application drafting and submission via the CDSCO MD Online Portal
- Post-license support for compliance and renewals
Our proven track record of over 500 successful licenses ensures you save time and avoid costly mistakes.
Getting Started with Your CDSCO License Application
Assess your device classification: Confirm the device is Class B under the relevant notification.
Register on the CDSCO MD Online Portal: Create your company profile and familiarize yourself with application forms.
Prepare the test license application (Form MD13): Begin the process to manufacture and test samples.
Engage a notified body for audit planning: Early engagement accelerates scheduling.
Compile your Device and Plant Master Files: Use our comprehensive Device Master File guide and Plant Master File guide.
Schedule product testing: Contact CDSCO-approved labs promptly.
Submit the MD5 license application (Form MD3): Once testing and documentation are complete.
Starting early and leveraging expert guidance ensures your Retention Type Balloon Catheter obtains CDSCO approval efficiently, enabling you to confidently enter the Indian market.
For personalized assistance, contact us to initiate your licensing journey.