CDSCO License for Spring-loaded pneumoperitoneum needle, single-use
Medical Device Information
Intended Use
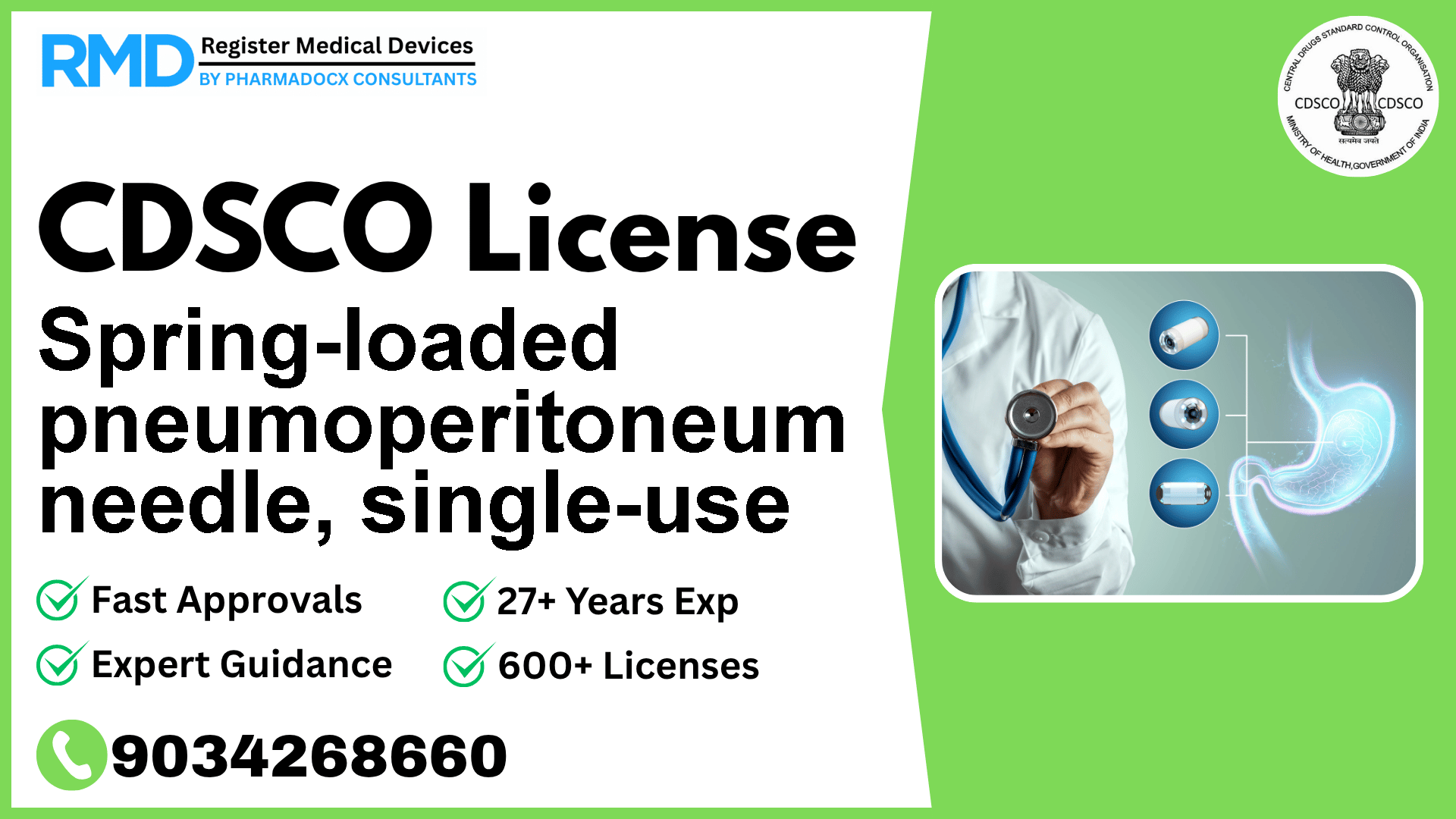
A slender, sharply-pointed metal tube designed to introduce or remove gas from the peritoneal cavity as a therapeutic or surgical/radiological procedural method. It is often inserted into the peritoneal cavity for the purpose of insufflation [e.g., with carbon dioxide (CO2)] to establish pneumoperitoneum prior to abdominal endoscopy.
Comprehensive Guide to CDSCO Licensing for Spring-Loaded Pneumoperitoneum Needle (Class B Medical Device)
As a medical device manufacturer or importer specializing in gastroenterology instruments, obtaining the correct regulatory approval is critical for market entry in India. The spring-loaded pneumoperitoneum needle, a Class B device, plays a pivotal role in minimally invasive abdominal procedures such as laparoscopies. With over 25 years of experience and having supported 500+ companies with CDSCO registrations, we provide an in-depth walkthrough of the licensing process tailored specifically to this device.
Understanding the Spring-Loaded Pneumoperitoneum Needle and Its Regulatory Significance
This single-use, spring-loaded needle is designed to safely and effectively introduce or remove gas (typically CO2) into the peritoneal cavity to create pneumoperitoneum – a prerequisite for many abdominal endoscopic procedures. Due to its invasive nature and direct interaction with internal bodily cavities, the device falls under Class B risk classification according to Indian medical device norms. Compliance with CDSCO regulations is mandatory to ensure patient safety and gain legal market access.
CDSCO Regulatory Framework for Class B Gastroenterology Devices
The Central Drugs Standard Control Organization (CDSCO) classifies medical devices into four risk categories: Class A (low risk), B (low-moderate risk), C (moderate-high risk), and D (high risk). Your spring-loaded pneumoperitoneum needle is a Class B device per Notification 29/Misc./03/2020-DC (182) dated 27.09.2021.
Class B devices require a manufacturing license known as the MD5 license issued by the respective State Licensing Authority. Importers must obtain the MD15 import license from CDSCO’s Central Licensing Authority.
Risk Classification and License Requirements for Spring-Loaded Pneumoperitoneum Needle
| Risk Class | License Type | Issuing Authority | Typical Duration | Fees (INR) |
|---|---|---|---|---|
| Class B | MD5 License | State Authority | 3-4 months | 5000 + 500 per product |
| Import | MD15 License | Central Authority | 5-6 months | Varies by class and product |
This device’s Class B status means you must first secure a test license (MD13) prior to manufacturing license application.
Step-by-Step Manufacturing License Process for MD5 (Form MD3)
Test License (Form MD13): Before full manufacturing license application, obtain a test license from the State Licensing Authority. This takes approximately 1.5-2 months.
Product Testing: Submit your spring-loaded pneumoperitoneum needle to government-approved testing laboratories for compliance analysis. Refer to the CDSCO-approved Testing Laboratories list for selection.
Document Preparation: Assemble your technical documentation including Device Master File, Plant Master File, Risk Management File, Essential Principles Checklist, and Quality Management System documents.
Application Submission: File the MD5 manufacturing license application using Form MD3 on the CDSCO MD Online Portal.
Audit by Notified Body: Upon application acceptance, a notified body (refer to the Notified Bodies List) will conduct an on-site audit of your manufacturing facility.
Query Resolution: Address any queries raised by the licensing authority or notified body promptly to avoid delays.
License Grant: Once all criteria are met, the MD5 license is granted on Form MD5, allowing legal manufacture of the device.
Essential Documents for MD5 Manufacturing License Application
- Company Constitution (MOA/AOA)
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Qualification and Experience Details of Technical Staff
- Fire and Pollution NOCs
- Device Master File (DMF) detailing design, specifications, and safety features (Device Master File Guide)
- Plant Master File (PMF) describing manufacturing processes and controls (Plant Master File Guide)
- Essential Principles Checklist aligned with Schedule I of Medical Device Rules
- Risk Management File specific to the pneumoperitoneum needle (Risk Management)
- Test Reports from accredited laboratories
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documents (ISO 13485 certification recommended)
Import License Process for MD15 (Form MD14) for Spring-Loaded Pneumoperitoneum Needle
If you are importing this Class B device, the MD15 license is mandatory, issued by CDSCO Central Licensing Authority. Unlike manufacturing, no test license is required upfront.
Steps:
Document Compilation: Prepare documents including manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device and Plant Master Files, Wholesale License, and Company Constitution.
Application Submission: Submit the import license application (Form MD14) through the CDSCO MD Online Portal.
Query Handling: Respond promptly to any departmental queries.
License Issuance: Upon satisfactory review, MD15 import license will be granted.
Import License Documents Required
- Manufacturing License of the device in the exporting country
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate (if available)
- Device Master File
- Plant Master File
- Wholesale License (if applicable)
- Company Constitution Documents
Timeline and Processing Duration for Spring-Loaded Pneumoperitoneum Needle
| License Type | Approximate Duration | Key Milestones |
|---|---|---|
| MD13 Test License | 1.5 to 2 months | Application to issuance |
| Product Testing | 1 to 1.5 months | Sample submission and report |
| MD5 Manufacturing License | 3 to 4 months (including above) | Audit and query resolution |
| MD15 Import License | 5 to 6 months | Document review and approval |
Government Fees and Cost Breakdown
For the spring-loaded pneumoperitoneum needle (Class B device):
MD5 Manufacturing License:
- Application Fee: ₹5,000
- Per Product Fee: ₹500
MD13 Test License:
- Usually included in the above process but may vary by state
MD15 Import License:
- Based on device class and product count; approximately 1,000 per product for Class B devices
Common Challenges and Practical Solutions
- Delay in Product Testing: Government-approved labs have limited slots. Pre-book testing and ensure sample readiness to avoid bottlenecks.
- Incomplete Documentation: Use checklists to verify all required documents before submission. Our Device Master File guide can assist in comprehensive preparation.
- Audit Non-Compliance: Conduct internal mock audits before notified body visits to ensure adherence to QMS and manufacturing standards.
- Query Resolution Delays: Assign a dedicated regulatory liaison to respond swiftly to queries from CDSCO or auditors.
Expert Consultation and Support
Navigating CDSCO licensing can be complex due to evolving regulations and procedural nuances. Our seasoned regulatory consultants have successfully guided over 500 companies through the licensing maze, minimizing common pitfalls and accelerating approvals. We offer:
- Customized documentation support
- End-to-end application filing on the CDSCO MD Online Portal
- Audit preparation and training
- Post-approval compliance monitoring
Getting Started with Your CDSCO License Application for Spring-Loaded Pneumoperitoneum Needle
- Assess Your Device Classification: Confirm the Class B status and applicable regulatory pathways.
- Prepare Your Documentation: Begin compiling your Device Master File and Plant Master File using expert templates.
- Apply for Test License (MD13): Submit your test license application via the CDSCO portal.
- Schedule Product Testing: Contact CDSCO-approved labs early to book testing slots.
- Plan for Audit: Identify and engage a notified body from the official Notified Bodies List.
- Submit Manufacturing License Application (MD5): Once testing and documentation are complete, file your application.
By following these practical steps and leveraging expert support, you can confidently navigate the CDSCO licensing process for your spring-loaded pneumoperitoneum needle and achieve timely market entry.
For detailed guidance tailored to your specific product and business model, contact our regulatory consulting team today and benefit from over two decades of industry expertise.