CDSCO License for Straight Catheter
Medical Device Information
Intended Use
It is used in patients with neurogenic bladder or spinal cord injury, lessens the risk of urinary tract infection
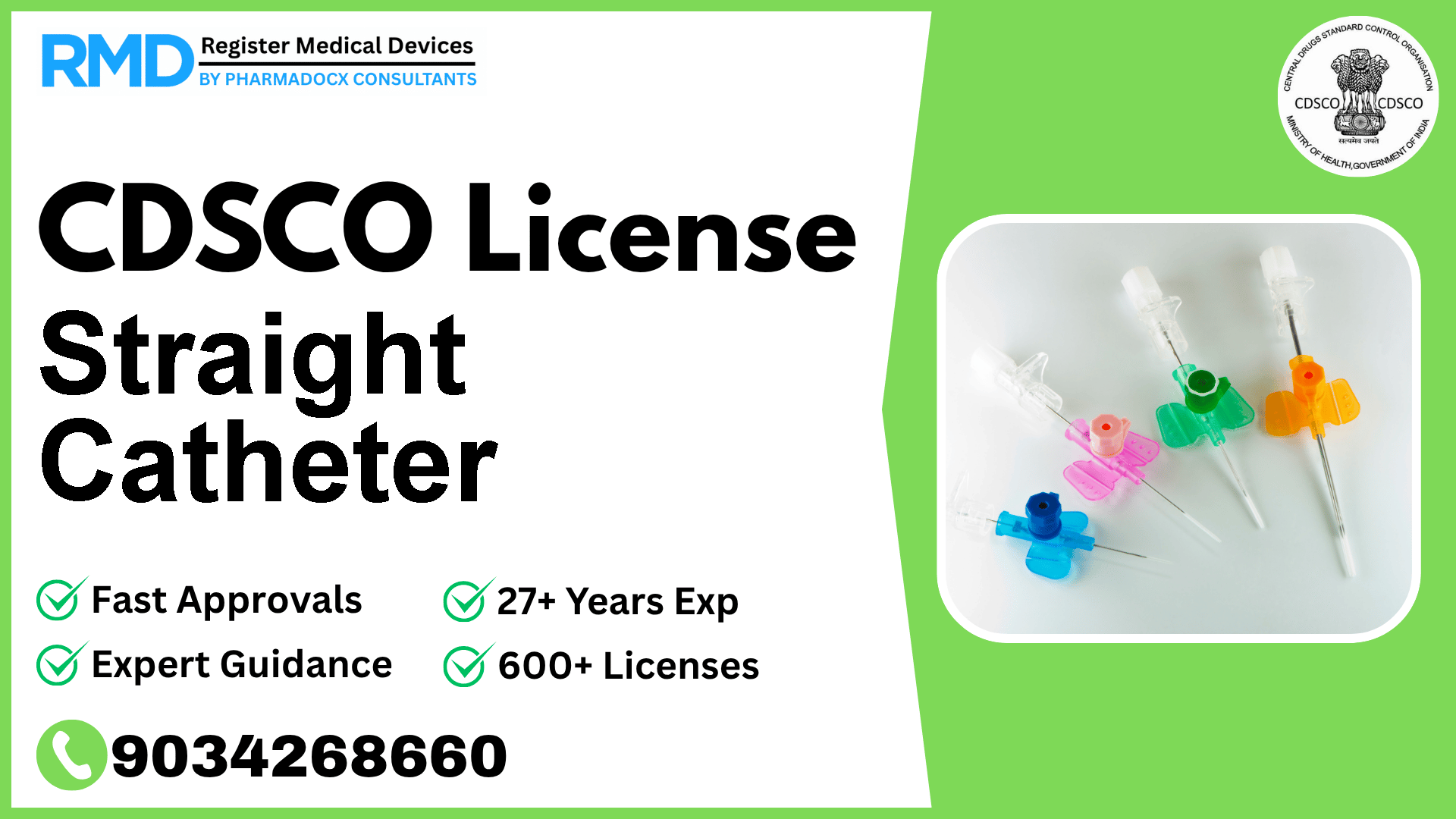
Comprehensive Guide to CDSCO Licensing for Straight Catheters (Class B Medical Device)
Straight catheters, essential medical devices used primarily for patients with neurogenic bladder or spinal cord injuries, play a critical role in reducing the risk of urinary tract infections through intermittent catheterization. As a regulated category under the Indian medical device framework, obtaining a CDSCO license is mandatory for manufacturing or importing straight catheters to ensure safety, efficacy, and compliance with Indian regulatory standards.
With over 25 years of experience and having supported 500+ companies, we provide a detailed walkthrough of the licensing process for straight catheters, classified as Class B devices under the CDSCO Medical Device Classification.
CDSCO Regulatory Framework for Straight Catheters
Straight catheters fall under the catheter category notified under Notification 29/Misc/3/2017-DC (292) dated 06.06.2018. The Central Drugs Standard Control Organization (CDSCO) regulates these devices through a risk-based classification system to ensure public health protection.
Being a Class B device, straight catheters require a manufacturing license under Form MD5, which is issued by the State Licensing Authority.
Risk Classification and License Requirements for Straight Catheters
- Risk Class: B (Low-Medium Risk)
- License Type: MD5 (Manufacturing License for Class A & B devices)
- Regulatory Authority: State Licensing Authority
- Applicable Forms:
- Test License: Form MD13
- Manufacturing License: Form MD3 (application), Form MD5 (grant of license)
Understanding this classification is crucial because it defines the documentation, testing, and audit requirements as well as the licensing authority and associated timelines.
Manufacturing License Process for Straight Catheters (MD5 License)
The MD5 licensing process involves multiple steps:
- Obtaining a Test License (Form MD13): A prerequisite for manufacturing license application, the test license allows you to manufacture samples for testing.
- Product Testing: Conduct product testing in CDSCO-approved laboratories to generate mandatory test reports.
- Document Preparation: Compile comprehensive documentation including Device Master File, Plant Master File, risk management files, and other statutory documents.
- Application Submission: File the manufacturing license application on Form MD3 through the CDSCO MD Online Portal.
- Audit by Notified Body: Engage an accredited notified body for site audit and technical review. You can check the list of notified bodies here.
- Query Resolution: Respond promptly to any queries from CDSCO or the notified body.
- License Grant: Upon successful compliance, receive the MD5 manufacturing license.
Manufacturing License Documents Required for Straight Catheters
The following documents are essential for the MD5 license application:
- Company Constitution Documents: Incorporation certificate, partnership deed, etc.
- Proof of Premises Ownership or Rent Agreement
- Technical Staff Qualification and Experience Proof
- Fire NOC and Pollution Control NOC
- Device Master File (DMF): Detailing design, manufacturing, and quality aspects. Our Device Master File guide elaborates on how to prepare this effectively.
- Plant Master File (PMF): Documenting facility capabilities and processes. See our Plant Master File guide for practical tips.
- Essential Principles Checklist: Demonstrating compliance with Indian medical device regulations.
- Risk Management File: Including hazard analysis and mitigation strategies. Learn more about medical device risk management.
- Test Reports: From CDSCO-approved testing laboratories. Refer to the Testing Laboratories list.
- Labels and Instructions for Use (IFU): Clear, compliant labeling and user manuals.
- Quality Management System (QMS) Documents: ISO 13485 certification and internal SOPs.
Import License Process for Straight Catheters (MD15 License)
If you are an importer rather than a manufacturer, you will require an Import License (Form MD15) from the Central Licensing Authority.
The import license process includes:
- Document preparation including manufacturing license of the foreign manufacturer, Free Sale Certificate, ISO 13485:2016, CE certificate, Device and Plant Master Files.
- Application submission on the CDSCO MD Online Portal.
- Query resolution.
- License grant.
Import licenses typically take 5-6 months and carry higher fees compared to manufacturing licenses.
Import License Documents Required
- Manufacturing License of foreign manufacturer
- Free Sale Certificate
- ISO 13485:2016 certification
- CE Certificate
- Device Master File
- Plant Master File
- Wholesale License
- Company Constitution Documents
Timeline and Processing Duration
- Test License (MD13): Approximately 1.5 to 2 months
- Product Testing: 2-3 weeks depending on laboratory schedules
- Manufacturing License Application (MD5): 3-4 months including audit and query resolution
Overall, expect a timeline of 3-4 months from test license application to final manufacturing license grant.
Government Fees and Costs
- Test License (MD13): Rs. 5000 approximately
- Manufacturing License (MD5): Rs. 5000 per application + Rs. 500 per product
For straight catheters, if you plan to license multiple variants, factor in the per-product fee accordingly.
Common Challenges and Solutions
Challenge 1: Delayed Testing and Reports Testing delays can stall your entire application. To mitigate this, plan testing early and select recognized CDSCO-approved labs from the Testing Laboratories list.
Challenge 2: Incomplete Documentation Missing or substandard documents cause repeated queries. Utilize expert templates for Device and Plant Master Files and ensure your QMS and risk files are up to date.
Challenge 3: Audit Non-Compliance Audits by notified bodies can be rigorous. Prepare by conducting internal audits and reviewing all processes against regulatory requirements. Refer to the list of notified bodies for selecting experienced auditors.
Challenge 4: Regulatory Updates and Changes Stay informed on CDSCO notifications and guidelines by subscribing to official updates and consulting with experienced regulatory consultants.
Expert Consultation and Support
Navigating CDSCO licensing can be complex, especially for Class B devices like straight catheters that require rigorous documentation and audits. Our team brings over 25 years of regulatory expertise and has successfully assisted 500+ medical device companies in India.
We offer end-to-end support:
- Gap analysis of existing documentation
- Preparation of Device and Plant Master Files
- Coordination with testing labs and notified bodies
- Application filing and query management
- Post-license compliance advisory
Getting Started with Your CDSCO License Application for Straight Catheters
- Evaluate Your Device Classification: Confirm your device falls under Class B as per CDSCO guidelines.
- Prepare Your Facility: Ensure your manufacturing site complies with GMP and has necessary infrastructure.
- Apply for Test License (MD13): Submit initial application via CDSCO MD Online Portal.
- Plan Product Testing: Schedule testing with a CDSCO-approved laboratory to avoid delays.
- Compile Documentation: Utilize our comprehensive guides on Device Master File and Plant Master File preparation.
- Schedule Notified Body Audit: Select an experienced notified body early from the official list.
- Submit MD5 License Application: File your complete application online and prepare for prompt query resolution.
By following these targeted steps and leveraging expert support, you can significantly streamline your CDSCO licensing journey for straight catheters and position your product for successful market entry in India.
For personalized guidance and comprehensive regulatory solutions, contact our expert team today.