CDSCO License for Suction system operated by vacuum
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A device used for such treatment as the aspiration of liquid or granular substances by using negative pressure supplied by the hospital's medical gas supply system at bed side for using on patient.
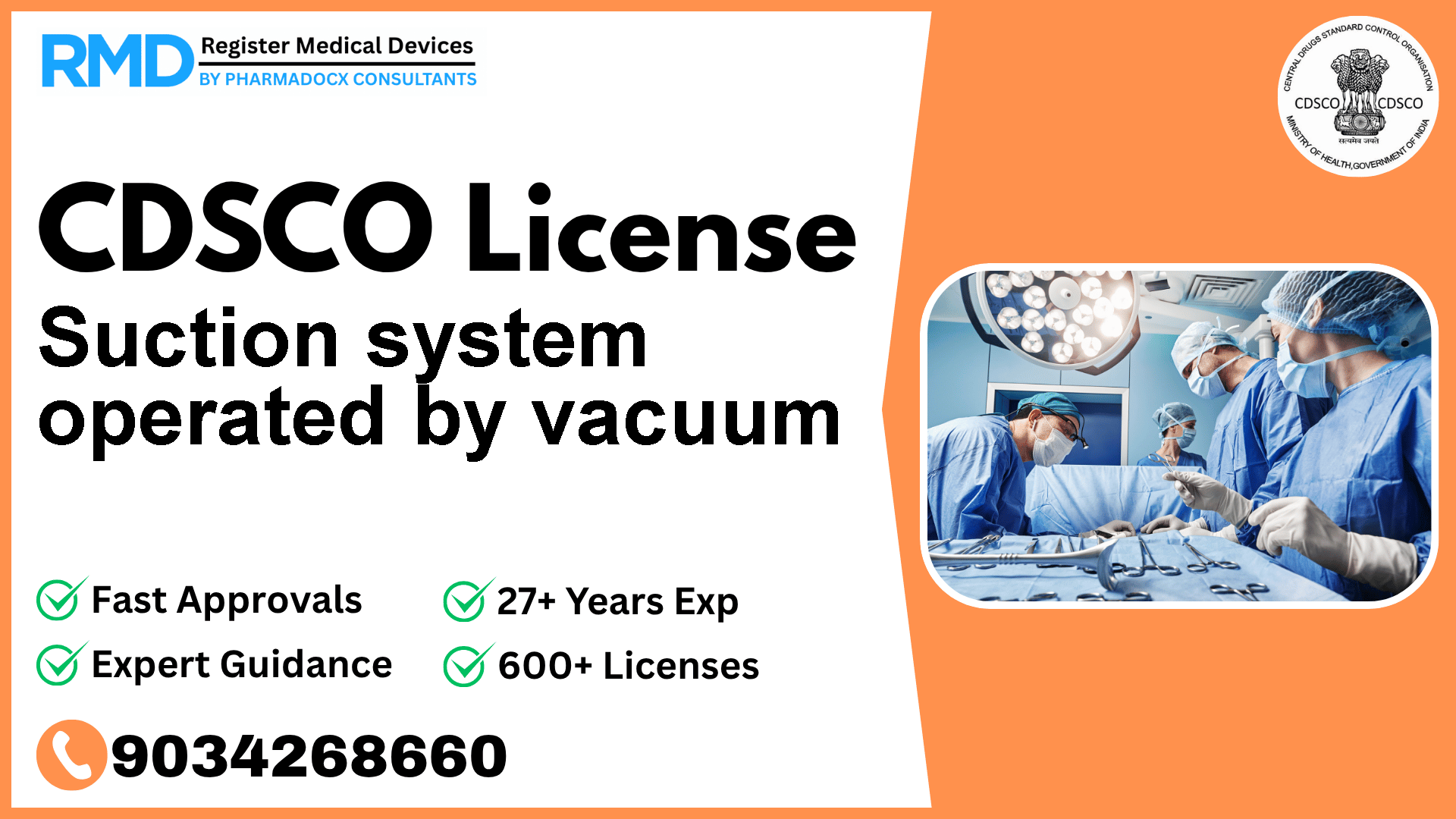
Comprehensive Guide to CDSCO Licensing for Suction Systems Operated by Vacuum (Class A Medical Device)
As a trusted regulatory consultancy with over 25 years of experience and having successfully assisted 500+ manufacturers and importers, we provide you with an in-depth, practical roadmap to obtaining your CDSCO license for a suction system operated by vacuum—a Class A medical device used in operation theatres for patient treatment by aspiration via negative pressure.
Understanding Your Device and Regulatory Importance
The suction system operated by vacuum is a critical device designed to aspirate liquids or granular substances at the bedside using the hospital's medical gas supply. Classified under Class A (low risk), this device requires strict compliance with CDSCO regulations to ensure safety and efficacy in clinical settings. Regulatory approval is mandatory before manufacturing or importing such devices into India.
CDSCO Regulatory Framework for Suction Systems (Class A)
Your device falls under the Operation Theatre category and is governed by the Ministry of Health and Family Welfare through the Central Drugs Standard Control Organization (CDSCO). The governing notification is File No. 29/Misc/03/2020-DC (199), dated 13.9.2021, which mandates manufacturers and importers to obtain an MD5 manufacturing license or MD15 import license respectively for Class A devices.
Risk Classification and License Requirements
According to CDSCO's Medical Device Rules, suction systems operated by vacuum are classified as Class A—the lowest risk category. For manufacturing, you must apply for an MD5 license (Form MD3) through the State Licensing Authority. Importers require the MD15 license from the Central Licensing Authority.
For a detailed overview of risk classification, we recommend reviewing the Medical Device Classification guide.
Manufacturing License Process (MD5) for Suction Systems
The manufacturing license process for Class A devices involves multiple steps:
Obtain Test License (Form MD13): Apply for a test license which typically takes 1.5 to 2 months. This temporary license allows you to conduct product testing.
Product Testing: Get your suction system tested by CDSCO-approved government laboratories. Refer to the Testing Laboratories list for authorized centers.
Document Preparation: Compile required technical and legal documents such as Device Master File, Plant Master File, Risk Management File, etc.
Submit Manufacturing License Application (Form MD3): Apply via the CDSCO MD Online Portal.
Audit by Notified Body: A notified body conducts a factory audit to verify compliance. Check the Notified Bodies list for authorized auditors.
Respond to Queries: Address any observations from the audit or CDSCO review.
Grant of MD5 License: Upon successful review, the license is issued in Form MD5.
Manufacturing License Documents Required
Preparing a comprehensive dossier is key to avoid delays. For your suction system, the following documents are essential:
- Company Constitution and Registration Proof
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualification and Experience Certificates
- Fire NOC and Pollution Control NOC
- Device Master File (DMF): Detailed device specifications, design, and manufacturing processes. Our Device Master File guide helps you create compliant DMFs.
- Plant Master File (PMF): Manufacturing facility details and quality systems. Refer to our Plant Master File guide.
- Essential Principles Checklist confirming compliance with safety standards
- Risk Management File demonstrating hazard identification and mitigation, per Risk Management practices
- Test Reports from government-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System (QMS) documentation (preferably ISO 13485 compliant)
Import License Process (MD15) Overview
If you plan to import the suction system, an MD15 license is mandatory. This is a central license with a processing time of 5-6 months. Import licensing requires:
- Valid manufacturing license from the country of origin
- Free Sale Certificate
- ISO 13485:2016 certificate
- CE Certificate or equivalent
- Device Master File and Plant Master File
- Wholesale license
- Company registration documents
Application is made on the CDSCO MD Online Portal using Form MD14.
Timeline and Processing Duration
For Manufacturing License (MD5):
- Test License (MD13): 1.5 to 2 months
- Product Testing: 1 month (varies by lab workload)
- Document preparation and application submission: 1 month
- Factory audit and queries resolution: 1 to 1.5 months
- Total Time: Approximately 3 to 4 months
For Import License (MD15):
- Document preparation and application: 1 month
- CDSCO review and queries: 4 to 5 months
- Total Time: Approximately 5 to 6 months
Government Fees and Costs
For your Class A device manufacturing license (MD5):
- Application fee: Rs 5,000 per application
- Product fee: Rs 500 per product
For import license (MD15):
- Site fee: USD 1,000
- Product fee: USD 50 per product
Note: Fees are subject to revision by CDSCO. Always check the latest fee structure on the official portal.
Common Challenges and Solutions
Challenge 1: Delay in product testing
- Solution: Pre-book testing slots at government-approved labs and prepare samples meticulously to avoid retesting.
Challenge 2: Incomplete documentation leading to queries
- Solution: Use checklists from official guidelines and consult expert regulatory consultants to ensure dossier completeness.
Challenge 3: Audit non-compliance
- Solution: Conduct internal mock audits and train your staff on CDSCO requirements beforehand.
Challenge 4: Navigating CDSCO portal complexities
- Solution: Familiarize yourself with the CDSCO MD Online Portal and maintain all documents in the required digital formats.
Expert Consultation and Support
Navigating CDSCO licensing can be complex, especially for newcomers. Our team has supported over 500 companies through the entire journey—from test license procurement to final license grant. We offer:
- Gap analysis of your current compliance status
- Preparation of Device and Plant Master Files
- Coordination with notified bodies and testing labs
- Assistance in audit preparation and post-audit responses
- End-to-end application filing and follow-up
Getting Started with Your CDSCO License Application
Step 1: Initiate your test license application (Form MD13) via the CDSCO MD Online Portal.
Step 2: Arrange for product testing at an authorized government laboratory.
Step 3: Simultaneously, start compiling your Device Master File and Plant Master File per CDSCO guidelines.
Step 4: Prepare your Quality Management System documentation aligned with ISO 13485.
Step 5: Once testing is complete, submit your MD5 license application (Form MD3) online.
Step 6: Coordinate with your notified body for the mandatory factory audit.
Step 7: Respond promptly and thoroughly to any queries raised during the evaluation process.
By following this structured approach, you can minimize delays and ensure a smooth licensing journey. Our expertise is at your disposal to guide you every step of the way—contact us to leverage 25+ years of regulatory excellence.
For further reading and detailed guides, explore our resources:
Your successful entry into the Indian medical device market begins with the right CDSCO license. Let us help you achieve it efficiently and compliantly.