CDSCO License for Tracheal prosthesis
Medical Device Information
Intended Use
It is intended to be implanted to restore the structure and/or function of the trachea or trachealbronchial tree
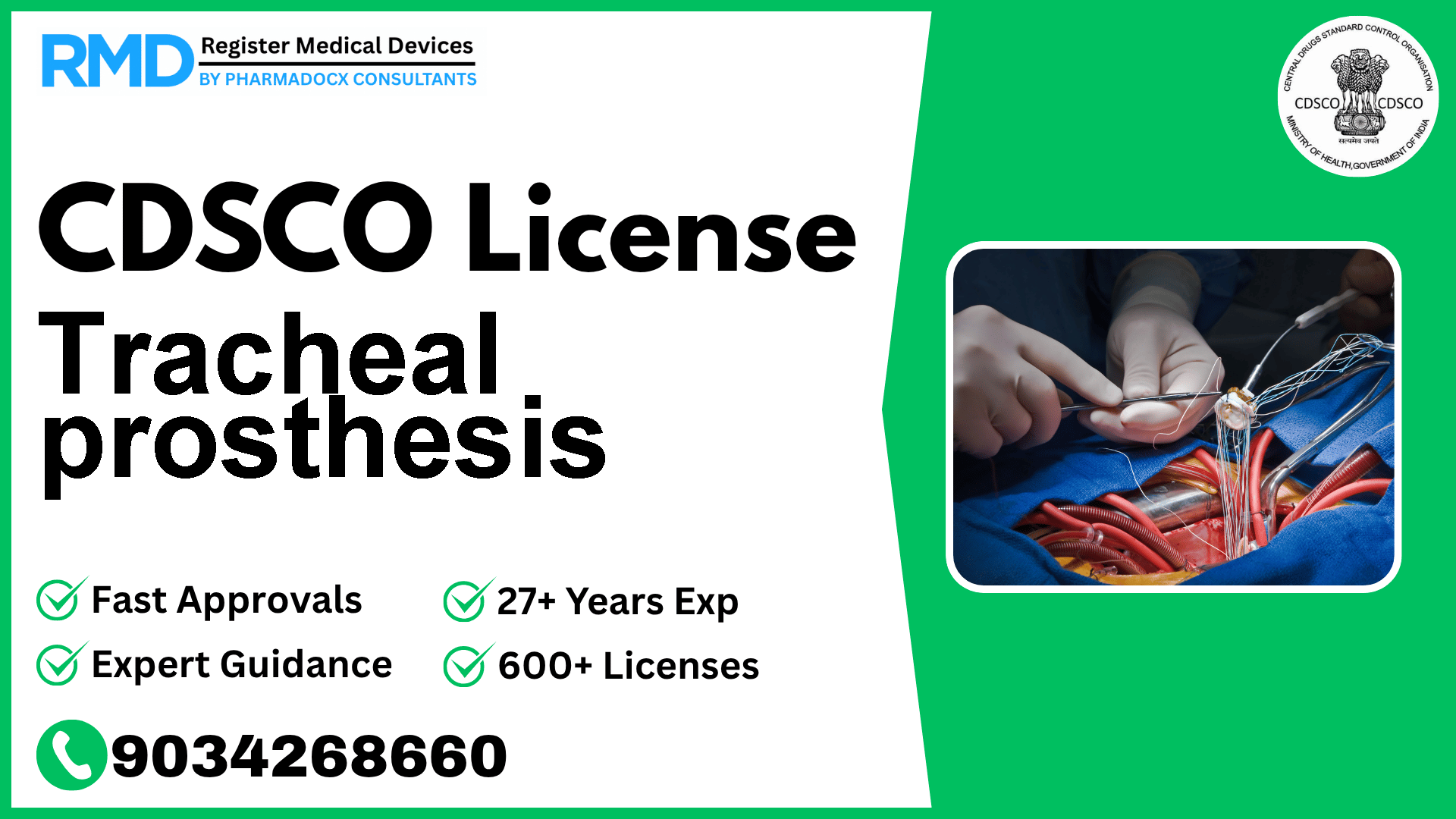
Comprehensive Guide to CDSCO Licensing for Tracheal Prosthesis (Class C Medical Device)
Navigating the regulatory landscape for medical devices in India can be complex, especially for high-risk implants like the tracheal prosthesis. With over 25 years of experience and having supported 500+ companies in obtaining CDSCO licenses, we provide you an expert, step-by-step guide tailored specifically to the Class C tracheal prosthesis—a critical internal prosthetic replacement designed to restore the tracheal or tracheobronchial tree structure and function.
Understanding the Tracheal Prosthesis and Its Regulatory Importance
A tracheal prosthesis is an implantable medical device used to rebuild or substitute a segment of the trachea, ensuring airway patency and function. Because it directly interfaces with critical respiratory structures, it is classified as a Class C device under CDSCO regulations, implying moderate to high risk. Proper licensing ensures compliance with safety, quality, and efficacy standards, protecting patients and enabling seamless market entry in India.
CDSCO Regulatory Framework for Tracheal Prosthesis
The regulatory oversight of tracheal prostheses is governed by the Central Drugs Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare. According to Notification 29/Misc/3/2017-DC (292) dated 06.06.2018, this device falls under Class C and requires a manufacturing license via MD9 and an import license via MD15 (if applicable). The framework mandates rigorous testing, documentation, and audits to uphold patient safety.
Risk Classification and License Requirements
- Risk Class: C (Moderate to High Risk)
- License Type for Manufacturing: MD9 License (Form MD7)
- Licensing Authority: Central Licensing Authority (CDSCO HQ)
- Approximate Timeline: 4-5 months (including testing, audit, and query resolution)
For importers, an MD15 Import License is mandatory.
Manufacturing License Process for MD9 (Class C Devices)
- Test License (Form MD13): Before full manufacturing license application, obtain a test license to manufacture limited quantities for testing purposes. This process takes approximately 1.5-2 months.
- Product Testing: Conduct mandatory product testing at CDSCO-approved testing laboratories to verify compliance with Indian standards. The CDSCO Testing Laboratories list can help identify authorized labs.
- Document Preparation: Compile a comprehensive dossier including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, Test Reports, and quality management system documents.
- License Application (Form MD7): Submit the application through the CDSCO MD Online Portal, attaching all required documents.
- Audit by CDSCO Inspectors: Post submission, CDSCO will schedule an inspection to verify compliance with Good Manufacturing Practices (GMP).
- Query Resolution: Address any observations or queries raised during the inspection or document review.
- Grant of License (Form MD9): Upon satisfactory completion, CDSCO issues the manufacturing license.
Manufacturing License Documents Required for Tracheal Prosthesis
- Company Constitution and Incorporation Certificates
- Proof of Ownership or Lease of Manufacturing Premises
- Qualification and Experience Documents of Technical Staff
- Fire and Pollution NOCs
- Device Master File (DMF) detailing design, manufacturing processes, and performance data (see our Device Master File guide)
- Plant Master File (PMF) outlining the manufacturing facility (refer to our Plant Master File guide)
- Essential Principles Compliance Checklist
- Risk Management File demonstrating hazard identification, risk analysis, and mitigation (our Risk Management guide can assist)
- Product Test Reports from CDSCO-approved labs
- Device Labeling and Instructions for Use (IFU)
- Quality Management System (QMS) Documents, preferably ISO 13485:2016 certification
Import License Process for Tracheal Prosthesis (MD15)
For importers aiming to bring tracheal prostheses into India, the import license is granted by the Central Licensing Authority on Form MD14, leading to the issuance of an MD15 license.
Key steps include:
- Document Compilation: Gather manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate (if applicable), Device Master File, Plant Master File, Wholesale License, and Company Constitution.
- Application Submission: Apply via the CDSCO MD Online Portal.
- Review and Query Resolution: Respond promptly to any department queries.
- Grant of License: Typically completed within 5-6 months.
Import License Documents Required
- Valid Manufacturing License from Country of Origin
- Free Sale Certificate
- ISO 13485:2016 and CE Certificates
- Device Master File and Plant Master File
- Wholesale License for Distribution
- Company Constitution and Incorporation Documents
Timeline and Processing Duration
| License Type | Duration |
|---|---|
| Test License | 1.5-2 months |
| MD9 Manufacturing License | 4-5 months (total including test license) |
| MD15 Import License | 5-6 months |
Government Fees and Costs
- MD9 Manufacturing License: Rs 50,000 per application + Rs 1,000 per product
- MD15 Import License:
- Class C & D: USD 3,000 per site + USD 1,500 per product
Note: Fees are payable online via the CDSCO portal and may vary based on exchange rate fluctuations for import licenses.
Common Challenges and Solutions
- Delayed Testing: Testing at government-approved labs can bottleneck timelines. To mitigate this, schedule tests early and consider labs with shorter turnaround times from the official labs list.
- Incomplete Documentation: Missing or inconsistent Device Master Files or Risk Management documentation often lead to queries. Engage regulatory experts or consult our detailed guides to prepare thorough dossiers.
- Audit Non-compliance: Facilities not adhering to GMP face inspection failures. Conduct internal audits and staff training before CDSCO visits.
- Query Resolution Delays: Rapid and clear responses to CDSCO queries expedite approvals. Maintain a dedicated compliance team for prompt handling.
Expert Consultation and Support
Our extensive experience in successfully navigating CDSCO regulations for Class C devices like tracheal prostheses ensures you avoid common pitfalls and accelerate market entry. We offer end-to-end support—from test license acquisition, document preparation, audit facilitation, to import license processing.
Getting Started with Your CDSCO License Application
- Assess Your Device Classification: Confirm your device as Class C by referring to the Medical Device Classification guide.
- Initiate Test License Application: Apply for Form MD13 on the CDSCO MD Online Portal to begin manufacturing for testing.
- Plan for Product Testing: Coordinate with CDSCO-approved labs early.
- Prepare Documentation: Start compiling Device Master File, Plant Master File, and Risk Management documentation.
- Engage Notified Bodies: For audit readiness, identify notified bodies recognized by CDSCO.
By following these actionable steps and leveraging our expertise, manufacturers and importers of tracheal prostheses can efficiently navigate the CDSCO licensing process and confidently enter the Indian healthcare market.
For personalized guidance and turnkey regulatory solutions, feel free to reach out to our expert team.