CDSCO License for Uterine manipulator, reusable
Medical Device Information
Intended Use
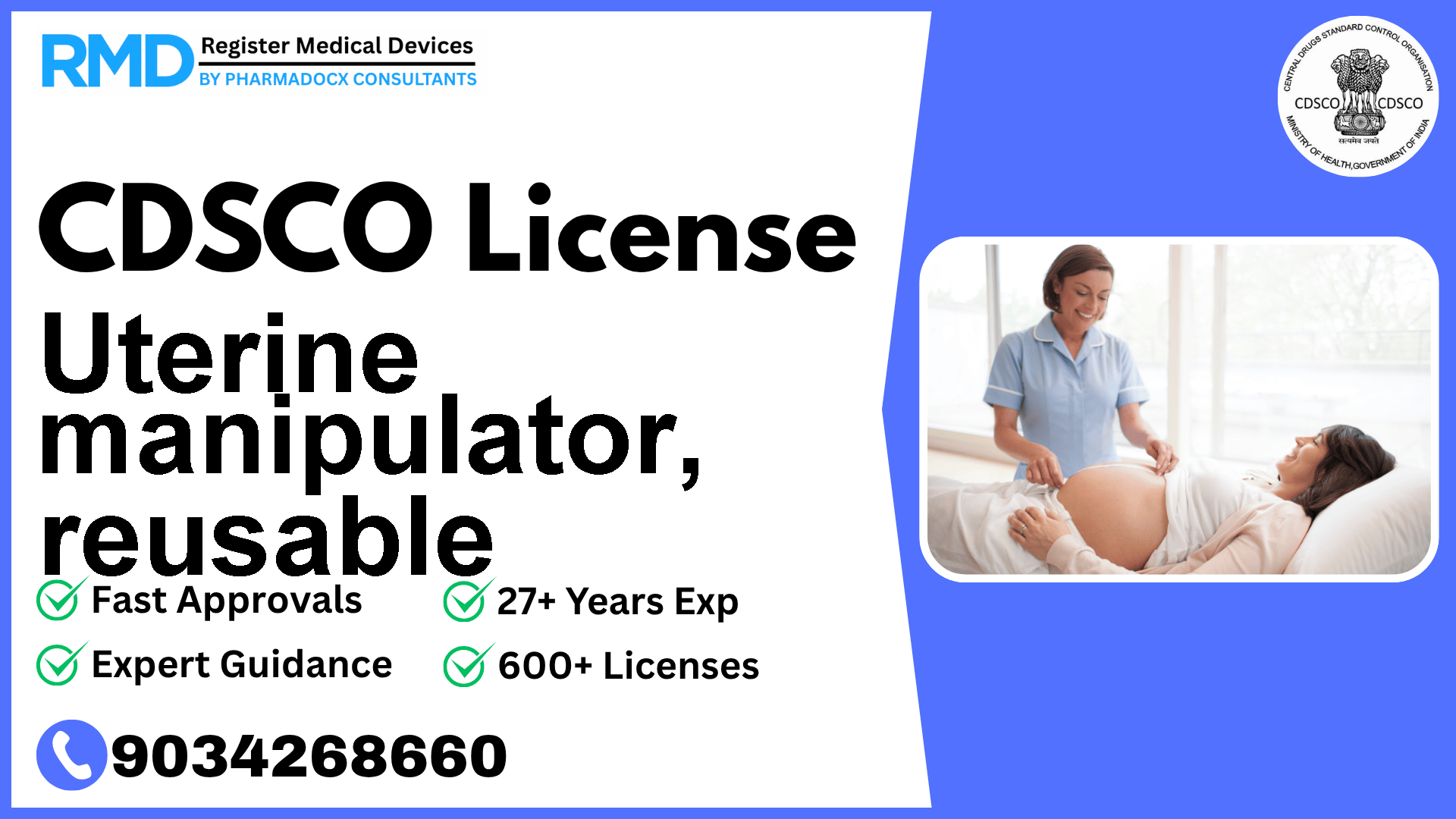
Surgical instrument designed to mechanically manipulate the position of the uterus during a gynaecological intervention
Comprehensive Guide to CDSCO Licensing for Reusable Uterine Manipulators (Class B Medical Device)
Navigating the regulatory landscape for medical devices in India can be complex, especially for specialized surgical instruments like the reusable uterine manipulator. As a trusted regulatory consultancy with over 25 years of experience and having supported 500+ companies, we provide you with a detailed, step-by-step roadmap to secure your CDSCO license with confidence and compliance.
Understanding the Uterine Manipulator and Its Regulatory Significance
The reusable uterine manipulator is a critical surgical instrument designed to mechanically adjust the uterus’s position during gynecological procedures. Classified under Obstetrical and Gynecological devices, it falls into Risk Class B as per CDSCO’s risk classification system. This classification mandates a specific regulatory pathway ensuring patient safety and device efficacy.
Given its reuse nature and direct surgical application, adherence to stringent quality and regulatory standards is non-negotiable. Achieving CDSCO approval not only assures compliance with India’s medical device regulations but also facilitates trust among healthcare providers and end-users.
CDSCO Regulatory Framework for Class B Medical Devices
The Central Drugs Standard Control Organization (CDSCO) governs medical device licensing in India. For Class B devices like the reusable uterine manipulator, the manufacturing license is granted by the State Licensing Authority under the MD5 license scheme. The process integrates product testing, document verification, and facility audits.
Manufacturers must comply with the latest notifications, including File No. 29/Misc./03/2020-DC (181) dated 03.6.2022, which specifically addresses classification and regulatory requirements for this device category.
Risk Classification and License Requirements for Uterine Manipulators
- Device Name: Uterine manipulator, reusable
- Risk Class: B (Moderate risk)
- Regulatory License: MD5 Manufacturing License (Application Form MD3)
- Authority: State Licensing Authority
This classification requires manufacturers to obtain a test license (MD13) before applying for the manufacturing license. The license ensures compliance with essential principles, quality management systems, and risk management protocols.
Learn more about medical device classification.
Manufacturing License Process (MD5) for Class B Devices
- Obtain Test License (Form MD13): Initial step allowing product testing at government-approved laboratories. Duration: Approximately 1.5 to 2 months.
- Product Testing: Submit samples to notified testing laboratories for compliance verification. View notified testing labs.
- Document Preparation: Assemble required technical and quality documentation, including Device Master File and Plant Master File.
- Application Submission (Form MD3): File your manufacturing license application via the CDSCO MD Online Portal.
- Audit by Notified Body: A mandatory inspection to verify compliance with manufacturing and quality standards. Find your notified body here.
- Query Resolution: Address any observations or queries raised by the licensing authority or the notified body.
- Grant of License (Form MD5): Upon satisfactory compliance, the State Licensing Authority issues the manufacturing license.
Manufacturing License Documents Required
To streamline your application, ensure you have the following documentation ready:
- Company Constitution documents
- Proof of ownership or lease agreement for manufacturing premises
- Qualification and experience certificates of technical staff
- Fire No Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File detailing design, specifications, and performance (Device Master File Guide)
- Plant Master File describing manufacturing facilities and controls (Plant Master File Guide)
- Essential Principles Checklist ensuring compliance with CDSCO standards
- Risk Management File demonstrating hazard analysis and mitigation (Risk Management Guide)
- Product Test Reports from government-approved laboratories
- Product labels, packaging details, and Instructions for Use (IFU)
- Quality Management System (QMS) documents, preferably ISO 13485:2016 certification
Import License Process (MD15) for Uterine Manipulators
If you are an importer looking to bring reusable uterine manipulators into India, you must apply for an MD15 Import License, granted by the Central Licensing Authority.
Key steps include:
- Document preparation including Manufacturing License from the country of origin, Free Sale Certificate, ISO certification, CE Certificate, and QMS documentation.
- Application submission on Form MD14 via the CDSCO MD Online Portal.
- Resolution of departmental queries.
- License grant on Form MD15.
Import licensing generally takes 5-6 months.
Timeline and Processing Duration
Manufacturing License (MD5) for Class B Device:
- Test License (MD13): 1.5 to 2 months
- Product Testing: 1 to 1.5 months (parallel with audit preparation)
- Audit & Application Processing: 1.5 to 2 months
- Total Duration: Approximately 3 to 4 months
Adhering to timelines and proactive document readiness significantly speeds up the process.
Government Fees and Costs
- Application Fee: Rs. 5,000 per application
- Product Fee: Rs. 500 per product
Additional costs include:
- Notified body audit fees (varies by body)
- Testing laboratory charges
- Consultancy and documentation preparation (optional but recommended for first-time applicants)
Common Challenges and Practical Solutions
- Incomplete Documentation: Delays are often due to missing or improperly prepared files. Use our comprehensive checklists to ensure completeness.
- Testing Delays: Early engagement with government-approved labs avoids bottlenecks.
- Audit Non-Compliance: Pre-audit internal assessments and mock audits can prevent adverse findings.
- Query Management: Timely and clear responses to CDSCO queries expedite approvals.
Expert Consultation and Support
With a proven track record supporting over 500 companies, we offer tailored consultancy services covering:
- Regulatory strategy and device classification
- Documentation preparation including Device and Plant Master Files
- Coordinating product testing and audits
- Application submission and follow-up
Our expertise ensures your uterine manipulator’s CDSCO licensing journey is smooth, compliant, and timely.
Getting Started with Your CDSCO License Application
- Assess your device classification: Confirm your device is Class B under current CDSCO notifications.
- Prepare your technical files: Gather all essential documents, leveraging our expert guides on Device Master Files and Risk Management.
- Apply for the MD13 test license: Submit your initial application via the CDSCO MD Online Portal.
- Schedule product testing: Coordinate with approved laboratories early to avoid delays.
- Engage a notified body: Identify and appoint a notified body for your audit.
- Submit the MD5 application: Once testing and documentation are complete, file the manufacturing license application.
- Respond promptly to queries: Maintain open communication with authorities to facilitate smooth processing.
Starting your CDSCO licensing journey with a clear plan and expert guidance is crucial. Contact us today to partner with seasoned professionals dedicated to bringing your reusable uterine manipulator to the Indian market efficiently and compliantly.