CDSCO License for Vessel Dialator for percutaneous Catheterization
Medical Device Information
Intended Use
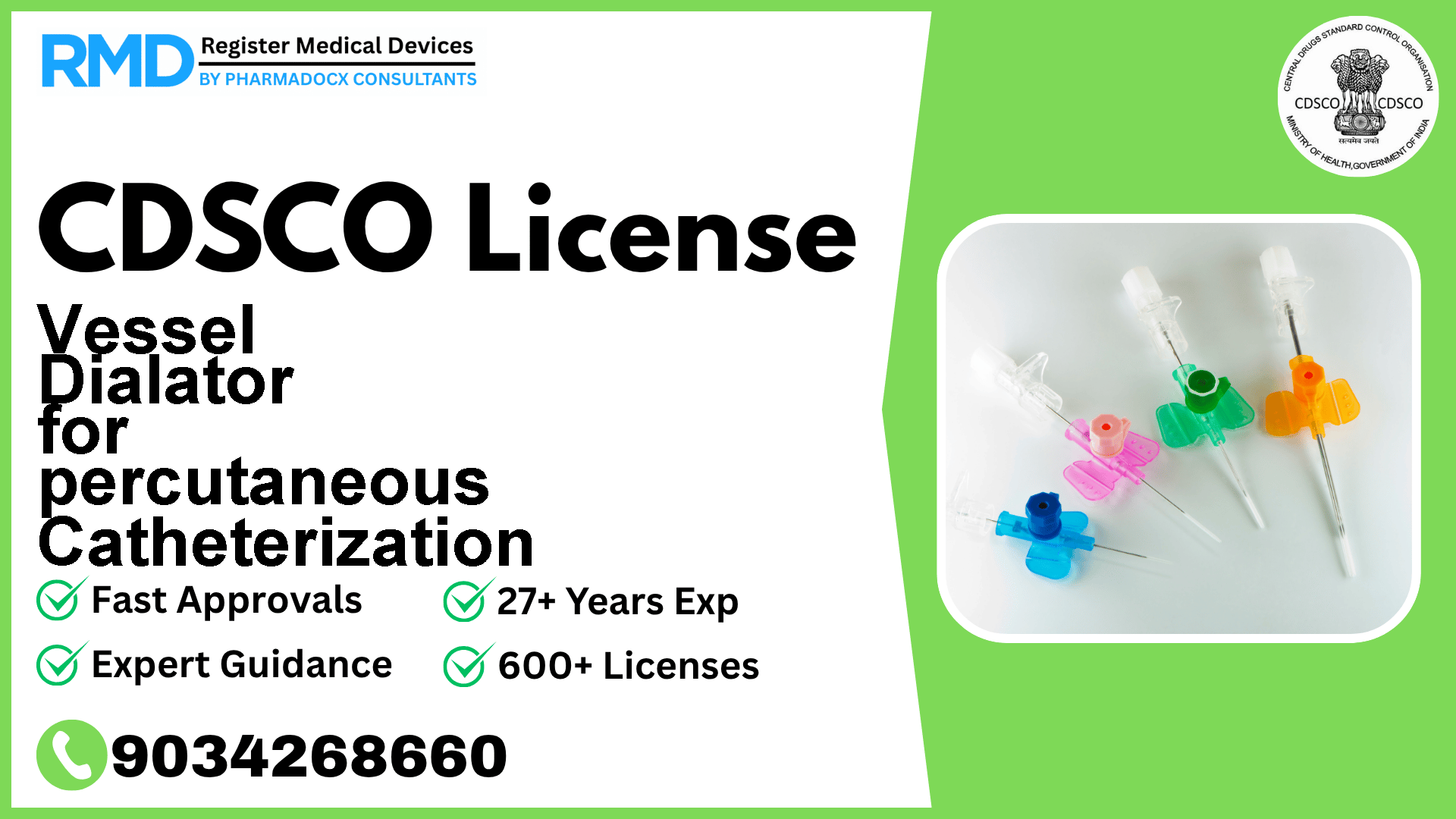
A vessel dilator for percutaneous catheterization is a device which is placed over the guide wire to enlarge the opening in the vessel, and which is then removed before sliding the catheter over the guide wire.
Comprehensive Guide to CDSCO Licensing for Vessel Dilator for Percutaneous Catheterization (Class B Device)
As seasoned regulatory consultants with over 25 years of experience and having successfully guided 500+ companies through the CDSCO licensing maze, we understand the challenges manufacturers and importers face when bringing a medical device like a Vessel Dilator for percutaneous catheterization to the Indian market. This vital catheter accessory, classified as a Class B device under the CDSCO framework, requires meticulous adherence to regulatory protocols to ensure timely market entry.
Understanding the Vessel Dilator and Its Regulatory Importance
A Vessel Dilator is designed to be placed over a guide wire to enlarge the vessel opening during percutaneous catheterization. It's removed before the catheter slides over the guide wire, playing a critical role in minimally invasive vascular procedures. Given its direct contact with the vascular system, regulatory authorities classify it as a Class B device, indicating a moderate risk profile that demands specific compliance measures.
CDSCO Regulatory Framework for Catheters – Class B Devices
Under the Medical Device Rules, 2017, catheters and associated accessories like vessel dilators fall into Class B when they pose moderate risk. The regulatory oversight for Class B devices is administered primarily at the State level through the CDSCO MD5 license system. This ensures that devices meet quality, safety, and performance benchmarks before market approval.
Risk Classification and License Requirements for Vessel Dilator
- Risk Class: B
- License Type: MD5 (Manufacturing License for Class A and B Medical Devices)
- Application Form: MD3
- Regulatory Authority: State Licensing Authority
The device notification number 29/Misc/3/2017-DC (292), dated 06.06.2018, officially includes vessel dilators under the catheter category, confirming the applicable regulatory path.
Manufacturing License Process (MD5) for Vessel Dilator
Obtaining the MD5 license is a multi-step process that typically spans 3 to 4 months and includes:
Test License (MD13): Initially, manufacturers must apply for a test license using Form MD13. This license allows sample production for testing and generally takes 1.5 to 2 months.
Product Testing: Samples of the vessel dilator must be tested at government-approved laboratories. Refer to the official list of testing laboratories to select an authorized facility.
Document Preparation: Comprehensive documentation including Device Master File, Plant Master File, and Risk Management File must be prepared.
Application Submission: Submit the manufacturing license application on Form MD3 via the CDSCO MD Online Portal.
Audit by Notified Body: A notified body conducts an on-site audit of the manufacturing site and quality systems. Check the list of notified bodies authorized to perform MD5 audits.
Queries Resolution: Address any queries or observations raised by the licensing authority or notified body promptly.
Grant of License: Upon satisfactory compliance, the MD5 license is issued on Form MD5.
Manufacturing License Documents Required
The documentation package must be thorough and precise. Key documents include:
- Company Constitution/Registration Certificate
- Proof of Ownership or Lease Deed of Manufacturing Premises
- Technical Staff Qualification and Experience Certificates
- Fire and Pollution No Objection Certificates (NOCs)
- Device Master File (DMF) detailing design and specifications (Learn more about DMFs)
- Plant Master File (PMF) describing manufacturing facilities (Plant Master File Guide)
- Essential Principles Checklist confirming compliance with Indian Medical Device Regulations
- Risk Management File demonstrating hazard identification and mitigation (Risk Management Insights)
- Test Reports from government-approved labs
- Device Labeling and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, typically ISO 13485:2016 compliant
Import License Process (MD15) for Vessel Dilator
For importers of vessel dilators, an MD15 license granted by the Central Licensing Authority is mandatory before marketing in India. The process involves:
- Preparation of detailed documentation including Manufacturing License from the country of origin, Free Sale Certificate, CE Certificate (if applicable), Device and Plant Master Files, and wholesale license.
- Application submission on Form MD14 through the CDSCO MD Online Portal.
- Queries resolution with the Central Authority.
- License grant on Form MD15, typically within 5 to 6 months.
Refer to our detailed Import License guide for comprehensive insights.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate or Certificate of Market Authorization
- ISO 13485:2016 Certificate
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License in India
- Company Constitution Documents
Timeline and Processing Duration
| Process Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 to 2 months |
| Product Testing | 3 to 4 weeks |
| Document Preparation | 2 to 3 weeks |
| Application Processing (MD5) | 1 to 1.5 months |
| Audit and Queries Resolution | 1 month |
| Total Estimated Time | 3 to 4 months |
Government Fees and Costs
- Test License Fee (MD13): Rs. 5000 per application
- MD5 License Fee: Rs. 5000 per application + Rs. 500 per product
These fees are payable online via the CDSCO MD Online Portal.
Common Challenges and Solutions
Challenge: Delays in product testing due to sample non-conformity.
- Solution: Pre-assess samples internally and conduct pre-testing at accredited labs before official submission.
Challenge: Incomplete or inconsistent documentation.
- Solution: Use standardized templates for Device and Plant Master Files and conduct internal audits prior to submission.
Challenge: Audit non-compliance issues.
- Solution: Engage with a notified body early to understand audit expectations and train staff accordingly.
Expert Consultation and Support
Navigating the CDSCO licensing process for a Class B device like the Vessel Dilator requires strategic planning and regulatory know-how. We provide personalized consulting services covering:
- Preparation of all required documentation
- Coordination with notified bodies and testing labs
- Application submission and follow-up
- Training and compliance audits
Our expertise has helped over 500 companies expedite approvals with minimal back-and-forth, ensuring market readiness and compliance.
Getting Started with Your CDSCO License Application
Assess Device Classification: Confirm your device falls under Class B as per the latest CDSCO notification.
Prepare Documentation: Begin compiling the Device Master File, Plant Master File, Risk Management File, and other technical documents.
Apply for Test License (MD13): Submit your test license application via the CDSCO MD Online Portal to initiate product testing.
Engage Testing Labs: Coordinate with government-approved testing laboratories to schedule product testing.
Plan for Audit: Select a notified body from the official list and prepare your manufacturing site.
Submit Manufacturing License Application (MD3): After successful testing, apply for the MD5 license on the portal.
Address Queries Promptly: Respond to any observations from the licensing authority or notified body to avoid delays.
Receive License and Commence Manufacturing: Once granted, you can legally manufacture and market your vessel dilator in India.
By following these detailed steps, backed by our regulatory expertise, manufacturers and importers can confidently navigate the CDSCO licensing process for their Vessel Dilator devices, ensuring compliance and successful market entry.
For more detailed insights and hands-on support, contact our regulatory consultancy team specialized in medical device licensing in India.