CDSCO License for Vial Adapter
Medical Device Information
Intended Use
It is indicated to allow multiple needleless access to injection medication vials for transfer or withdrawal of fluids from the vial.
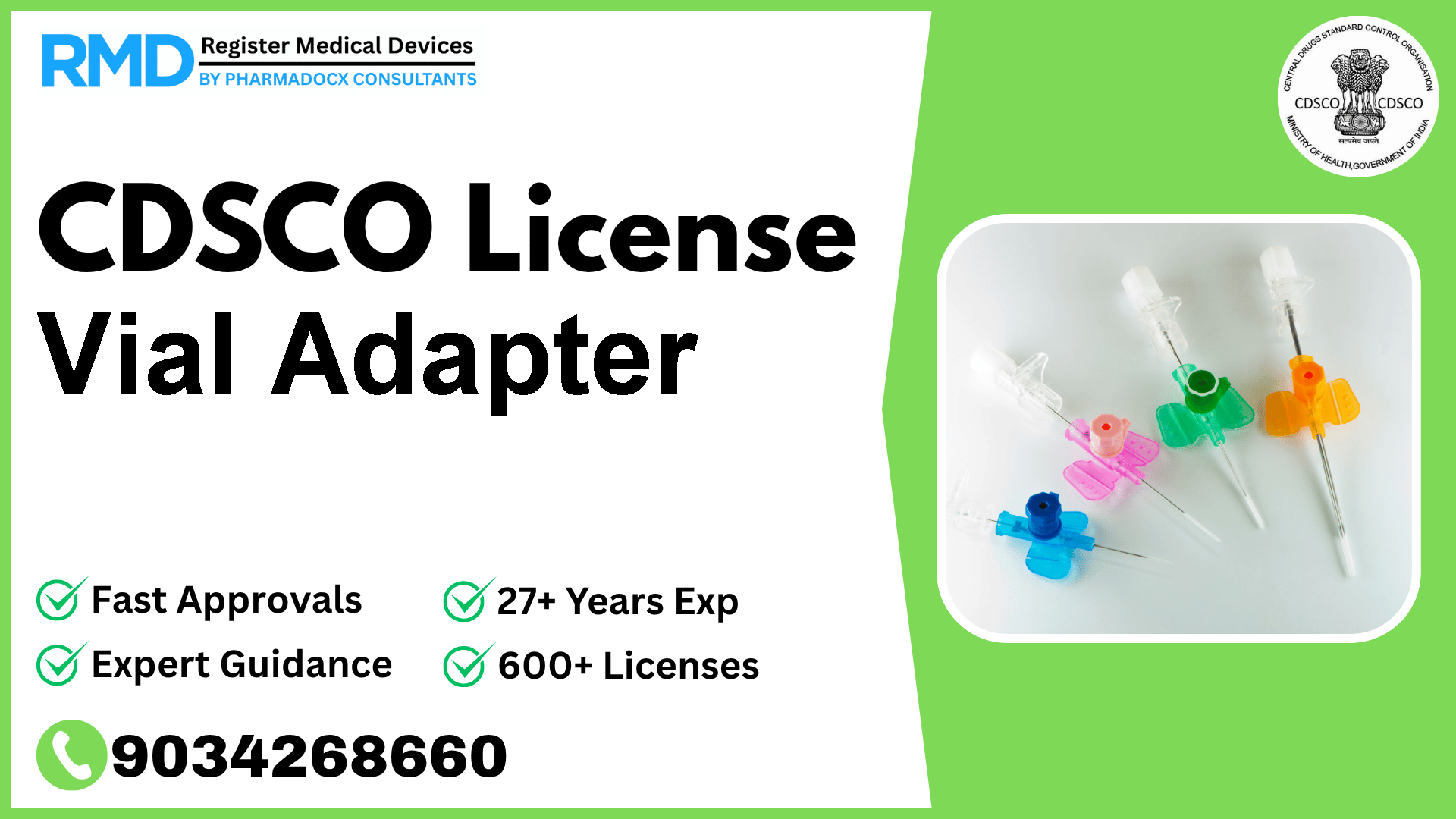
Comprehensive Guide to CDSCO Licensing for Vial Adapter (Class B Medical Device)
With over 25 years of experience assisting 500+ companies in securing CDSCO approvals, we understand the nuances and critical steps necessary for medical device manufacturers and importers. The Vial Adapter, classified under Risk Class B in the Catheters category, is a vital device that enables multiple needleless accesses to medication vials, facilitating fluid transfer or withdrawal. This device falls under the CDSCO Notification No. 29/Misc/3/2017-DC (292), notified on 06.06.2018.
CDSCO Regulatory Framework for Vial Adapters
In India, the Central Drugs Standard Control Organization (CDSCO) regulates medical devices under the Medical Device Rules 2017. Since the Vial Adapter is a Class B device, it requires a manufacturing license from the State Licensing Authority in the form of an MD5 license. For importers, an MD15 import license issued by CDSCO’s Central Licensing Authority is mandatory.
Risk Classification and License Requirements for Vial Adapter
The Vial Adapter is categorized as a Class B medical device, indicating moderate risk. According to the Medical Device Rules, Class A and B devices require the MD5 manufacturing license (Form MD3 application) issued by the State Authority. This classification dictates the scope, documentation, and audit requirements.
Manufacturing License Process for Vial Adapter (MD5 License)
Obtaining an MD5 license is a multi-step process involving several critical phases:
Test License Application (Form MD13): Before full manufacturing approval, you must obtain a test license, which takes approximately 1.5 to 2 months. This allows you to begin limited manufacturing and product testing.
Product Testing: The Vial Adapter must be tested at government-approved laboratories to ensure compliance with safety and performance standards. You can refer to the list of government testing laboratories.
Document Preparation and Submission: Compile all required documents and submit your application on the CDSCO MD Online Portal.
Notified Body Audit: After application submission, a notified body will conduct an audit of your manufacturing facility and quality systems. You can identify authorized bodies from the Notified Bodies List.
Query Resolution: Address any observations or queries from CDSCO or the notified body promptly.
Grant of MD5 License: Upon successful audit and document verification, the State Licensing Authority issues the manufacturing license.
Manufacturing License Documents Required for Vial Adapter
- Company Constitution (e.g., partnership deed, memorandum of association)
- Proof of ownership or lease of manufacturing premises
- Technical staff qualifications and experience certificates
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF) detailing design, specifications, and manufacturing processes (see our Device Master File Guide)
- Plant Master File (PMF) describing the facility and equipment (Plant Master File Guide)
- Essential Principles Checklist
- Risk Management File demonstrating compliance with ISO 14971 (Risk Management Guidance)
- Test Reports from government-approved labs
- Labels, packaging details, and Instructions for Use (IFU)
- Quality Management System (QMS) documentation, preferably ISO 13485:2016 certification
Import License Process for Vial Adapter (MD15 License)
For importers wishing to bring the Vial Adapter into India, the MD15 license is mandatory, issued by CDSCO Central Licensing Authority. The process typically takes 5-6 months and involves:
- Preparation of import license application with all required documents
- Submission through the CDSCO MD Online Portal
- Resolution of department queries
- Grant of MD15 license (Form MD15)
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate from the exporting country
- ISO 13485:2016 Certification
- CE Certificate or equivalent regulatory approval
- Device Master File and Plant Master File
- Wholesale Drug License (if applicable)
- Company Constitution and address proof
Timeline and Processing Duration
| License Type | Processing Time | Key Milestones |
|---|---|---|
| Test License (MD13) | 1.5 – 2 months | Application submission, lab testing approval |
| Manufacturing License (MD5) | 3 – 4 months | Audit, query resolution, license grant |
| Import License (MD15) | 5 – 6 months | Document verification, query resolution, license grant |
Government Fees and Costs
For the Vial Adapter, a Class B device, the fee structure is as follows:
MD5 Manufacturing License:
- Application Fee: Rs. 5,000
- Per Product Fee: Rs. 500
MD15 Import License:
- Site Fee: $2,000 per site
- Per Product Fee: $1,000
Additional costs include expenses for product testing, notified body audits, and document preparation.
Common Challenges and Practical Solutions
Delayed Document Preparation: Proper planning and early compilation of documents such as DMF, PMF, and Risk Management Files can prevent bottlenecks. Utilizing our expert guides ensures accuracy and completeness.
Audit Non-Compliance: Engage notified bodies early for pre-audit assessments to identify gaps in QMS or facility compliance.
Testing Delays: Schedule product testing well in advance with government-approved labs, considering their current workload.
Query Resolution: Maintain clear communication lines with CDSCO officers and promptly address any queries with detailed responses.
Expert Consultation and Support
Our team has successfully guided hundreds of manufacturers and importers through the CDSCO licensing maze. We offer:
- Comprehensive document preparation and review
- Coordination with notified bodies and testing laboratories
- End-to-end project management to meet timelines
- Tailored training on regulatory compliance and risk management
Getting Started with Your CDSCO License Application for Vial Adapter
Assess Your Device Classification: Confirm Vial Adapter’s Class B status and applicable license type (MD5 for manufacturing, MD15 for import).
Initiate Document Collection: Begin gathering all mandatory documents, including company and technical files.
Apply for Test License (MD13): Submit application on the CDSCO MD Online Portal to commence limited manufacturing and testing.
Plan Product Testing: Coordinate with government-approved labs early to avoid delays.
Schedule Notified Body Audit: Identify an accredited notified body from the official Notified Bodies List and prepare your facility accordingly.
Submit Manufacturing License Application (Form MD3): Once testing and audit are complete, file your MD5 license application online.
Prepare for Query Management: Stay responsive to CDSCO and notified body communications.
By following these actionable steps and leveraging our extensive expertise, your Vial Adapter can gain timely regulatory approval, enabling successful market entry in India.
For personalized assistance and turnkey solutions, reach out to our regulatory experts and ensure your CDSCO license journey is smooth and compliant.