CDSCO License for Video capsule endoscopy system capsule
Medical Device Information
Intended Use
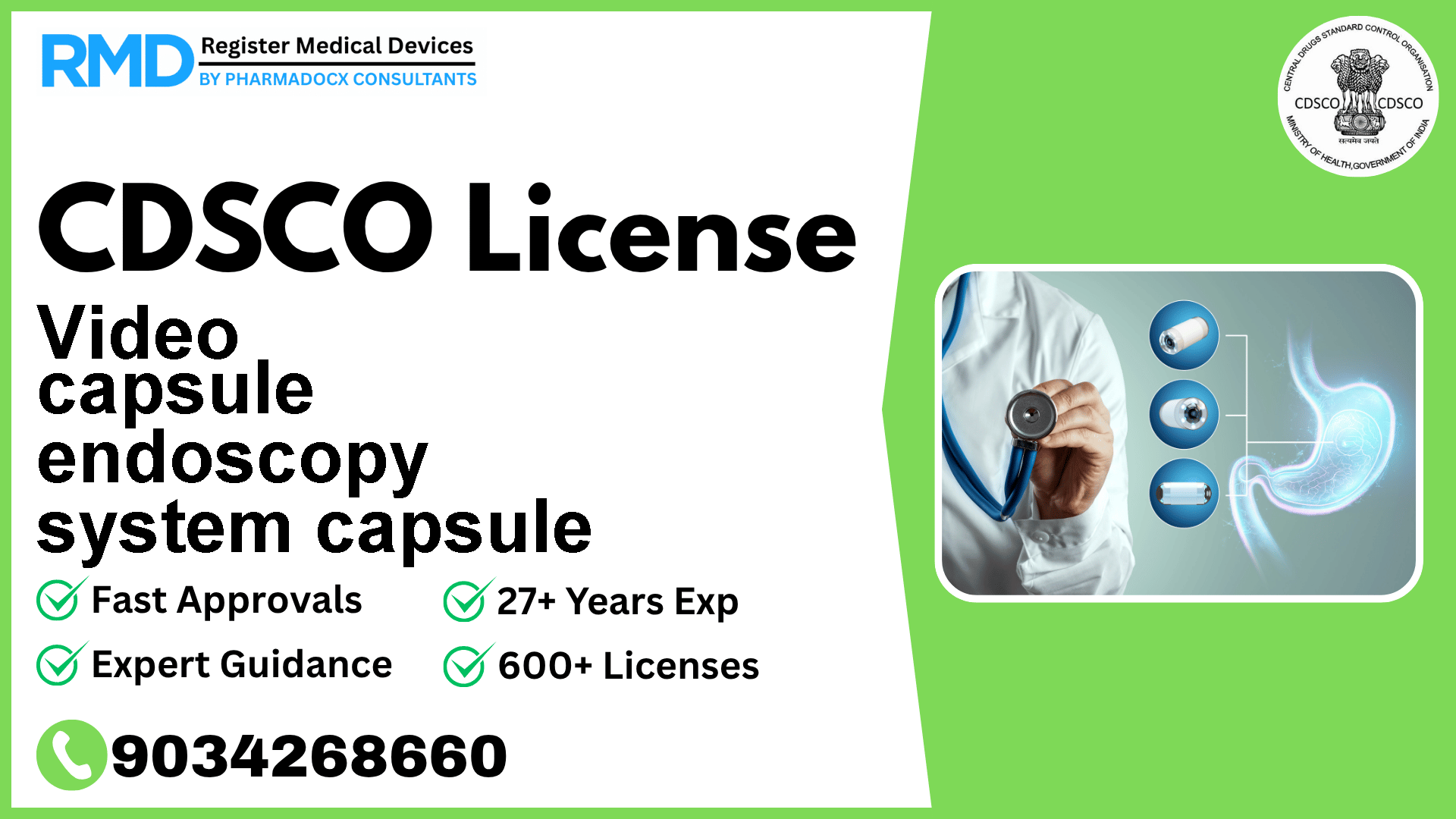
A non-sterile, battery-powered, electronic component device of a video capsule endoscopy system designed to be swallowed by a patient for the internal visualization and examination of the gastrointestinal (GI) tract.
Comprehensive Guide to CDSCO Licensing for Video Capsule Endoscopy System Capsule (Class B Medical Device)
As specialists with over 25 years of experience assisting more than 500 companies in navigating the complex regulatory landscape of India's medical device sector, we understand the unique challenges that manufacturers and importers face. If you're seeking to market a video capsule endoscopy system capsule—a non-sterile, battery-powered electronic device designed for internal visualization of the gastrointestinal tract—this guide will expertly walk you through the CDSCO licensing process tailored specifically for this Class B device.
Understanding the Video Capsule Endoscopy System Capsule and Its Regulatory Importance
Video capsule endoscopy capsules represent a breakthrough in gastroenterology diagnostics, providing a minimally invasive method for internal GI tract examination. Given its electronic nature and patient contact, regulatory compliance is critical to ensure safety, efficacy, and market access.
The Central Drugs Standard Control Organization (CDSCO) mandates licensing for all medical devices in India, with specific requirements based on risk classification. For your device, categorized as Class B, obtaining an MD5 manufacturing license is essential before production or distribution within India.
CDSCO Regulatory Framework for Class B Medical Devices
Under the Medical Device Rules, 2017, devices are categorized from Class A (low risk) to Class D (high risk). Your video capsule endoscopy system capsule falls under Class B, which demands a moderate level of regulatory scrutiny, including product testing, quality management system implementation, and facility audits.
The MD5 license is granted by the State Licensing Authority and governs manufacturing for Class A and B devices. This process ensures your device meets Indian safety and performance standards.
Risk Classification and License Requirements for Video Capsule Endoscopy Capsules
- Risk Class: B
- License Type: MD5 (Manufacturing License for Class A/B devices)
- Regulatory Authority: State Licensing Authority
- Applicable Notification: 29/Misc./03/2020-DC (182), dated 27.09.2021
Manufacturers must obtain a Test License (Form MD13) before applying for the MD5 license to facilitate product testing.
Step-by-Step Manufacturing License Process (MD5) for Class B Devices
- Obtain Test License (Form MD13): Initiate the process by applying for a test license, which typically takes 1.5 to 2 months. This license allows you to legally test your device.
- Product Testing: Get your capsule tested at government-approved laboratories to validate compliance with relevant standards. Refer to the list of testing laboratories as part of your preparation.
- Document Preparation: Compile all required documents, including technical files, quality management documents, and certifications.
- Submit Application for MD5 License (Form MD3): Apply through the CDSCO MD Online Portal, uploading all supporting documents.
- Facility Audit by Notified Body: A mandatory audit of your manufacturing premises is conducted by a notified body. You can check the list of notified bodies authorized for such audits.
- Respond to Queries: Address any queries raised by CDSCO or the notified body promptly to avoid delays.
- Grant of License (Form MD5): Upon satisfactory compliance, the manufacturing license is granted.
Essential Documents Required for MD5 License Application
To ensure a smooth application process for your video capsule endoscopy system capsule, prepare the following documents meticulously:
- Company Constitution and Incorporation Certificates (e.g., Memorandum & Articles of Association)
- Proof of Ownership or Lease of Manufacturing Premises
- Technical Staff Qualifications and Experience Certificates
- Fire NOC and Pollution Control NOC
- Device Master File (DMF): Detailed technical documentation covering design, manufacturing process, and product specifications. Our Device Master File guide provides comprehensive insights.
- Plant Master File (PMF): Documentation of manufacturing facility and quality systems. Learn how to create one effectively in our Plant Master File guide.
- Essential Principles Checklist: Demonstrating compliance with Indian medical device regulations.
- Risk Management File: Addressing risks associated with the device, aligned with international standards. See our Risk Management guide for best practices.
- Test Reports: From government-approved labs confirming conformance.
- Product Labels and Instructions for Use (IFU): Compliant with CDSCO labeling requirements.
- Quality Management System (QMS) Documentation: Typically ISO 13485:2016 certification and related procedures.
Import License Process (MD15) for Video Capsule Endoscopy Capsules
If you are an importer rather than a manufacturer, obtaining an MD15 import license is mandatory. This license is granted by the Central Licensing Authority and usually involves the following steps:
- Document preparation, including manufacturing license from country of origin, Free Sale Certificate, ISO 13485:2016, CE Certificate, Device and Plant Master Files, and wholesale license.
- Application submission on the CDSCO MD Online Portal.
- Resolution of any queries raised by the department.
- License grant typically within 5 to 6 months.
For detailed guidance, refer to our Import License guide.
Timeline and Processing Duration
| Process Stage | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Document Preparation | Variable (recommend 1 month) |
| MD5 License Application (Form MD3) | 1 – 1.5 months |
| Audit and Query Resolution | 1 month |
| Total Estimated Time | 3 – 4 months |
Government Fees and Costs
- Test License Fee (MD13): Included in the process, may vary by state
- MD5 License Fee: ₹5,000 per application + ₹500 per product
- Audit and Testing Costs: Variable; independent notified bodies and testing labs charge fees separately. Budget approximately ₹50,000-₹1,00,000 depending on scope.
Common Challenges and Practical Solutions
Challenge: Delays in test license issuance
- Solution: Submit a complete and accurate application with all mandatory documents upfront.
Challenge: Difficulty in sourcing notified bodies for audit
- Solution: Utilize the official notified bodies list to select authorized auditors early.
Challenge: Insufficient technical documentation
- Solution: Develop a detailed Device Master File and Risk Management File early in development. Our guides can help streamline this process.
Challenge: Non-compliance with labeling and IFU requirements
- Solution: Follow CDSCO guidelines strictly; conduct internal reviews and leverage expert consultancy.
Expert Consultation and Support
Navigating India's medical device regulatory environment can be daunting, especially for innovative devices like video capsule endoscopy systems. Our experienced regulatory consultants provide:
- Comprehensive document preparation and review
- Regulatory strategy tailored to Class B devices
- Coordination with testing labs and notified bodies
- Timely application submission and query handling
- Post-license compliance support
Partnering with experts who have a proven track record of 500+ successful CDSCO license approvals can significantly reduce your time-to-market and prevent costly mistakes.
Getting Started with Your CDSCO License Application for Video Capsule Endoscopy System Capsule
- Assess Your Device Classification: Confirm your device class as B using the Medical Device Classification tool.
- Register on the CDSCO MD Online Portal: Create your company profile and familiarize yourself with the application interface.
- Prepare Test License Application: Gather all documents for Form MD13 submission.
- Select Testing Laboratory and Notified Body: Identify suitable agencies early to schedule tests and audits.
- Compile Comprehensive Technical Documentation: Device Master File, Risk Management File, PMF, and QMS must be robust and audit-ready.
- Submit Test License Application: Begin the licensing timeline.
- Plan for Product Testing and Facility Audit: Coordinate timelines to avoid gaps.
- Prepare for Queries: Assign a dedicated team member to respond promptly.
- Apply for MD5 Manufacturing License: Upon successful test license and testing completion.
We encourage manufacturers and importers of video capsule endoscopy systems to start this process well in advance, as thorough preparation and expert guidance are key to securing timely CDSCO licensing.
For personalized assistance, feel free to contact our regulatory team who can provide end-to-end support tailored to your device and business needs.