CDSCO License for Video capsule endoscopy system
Medical Device Information
Intended Use
An assembly of electronic devices designed for the internal visualization and examination of sections of the gastrointestinal (GI) tract using a non-digestible video capsule after it has been swallowed by a patient.
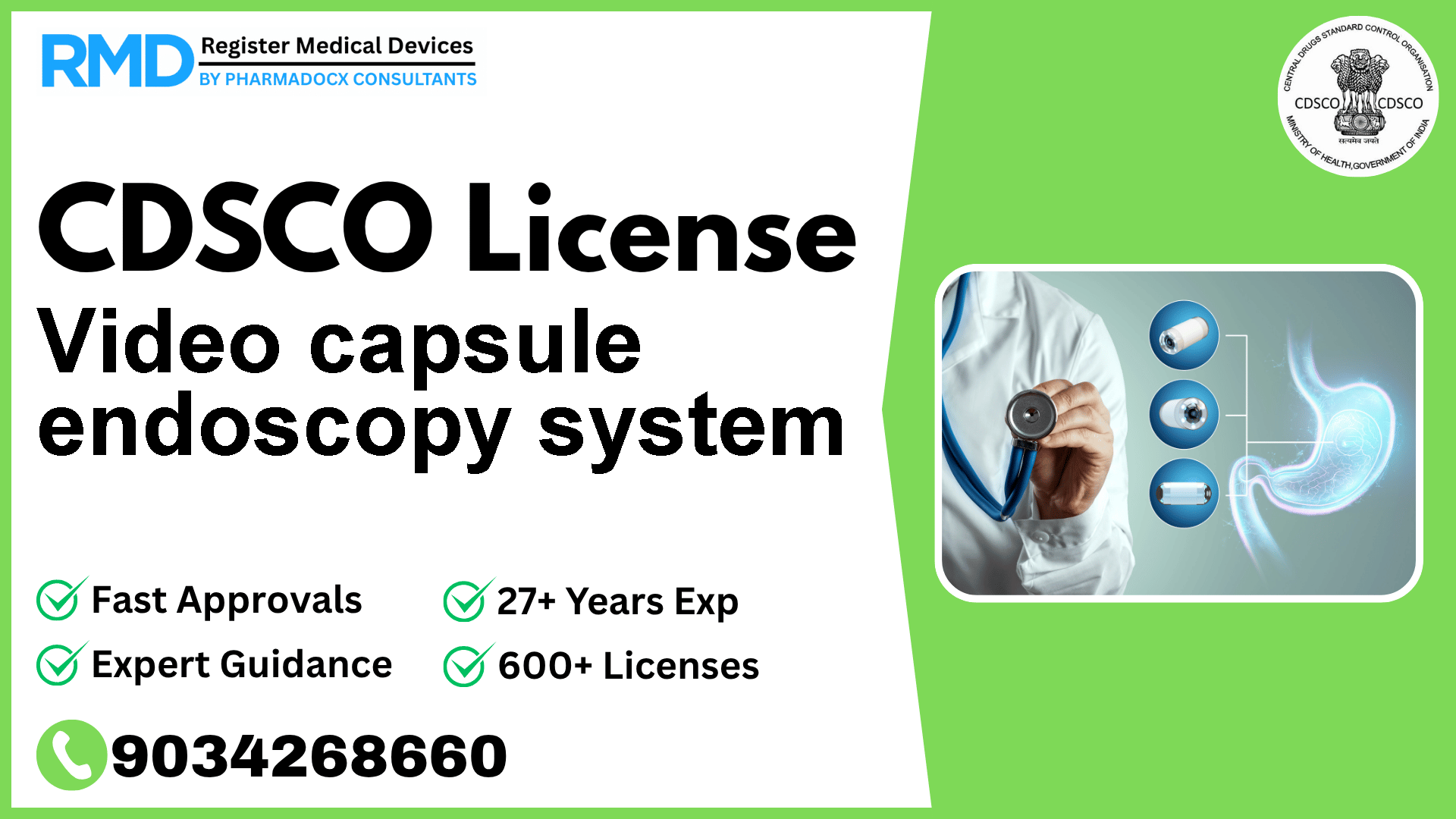
Comprehensive Guide to CDSCO Licensing for Video Capsule Endoscopy Systems (Class B Medical Device)
As specialists with over 25 years of experience assisting 500+ companies in navigating India’s medical device regulations, we understand the complexities manufacturers and importers face when launching sophisticated devices like the Video Capsule Endoscopy System. This revolutionary gastroenterology device allows non-invasive visualization of the gastrointestinal (GI) tract through a swallowed video capsule, classified as a Class B medical device under CDSCO’s framework.
Understanding the Video Capsule Endoscopy System and Its Regulatory Importance
The Video Capsule Endoscopy System is an assembly of electronic components designed to capture and transmit images from the GI tract, aiding in diagnosis without the need for traditional invasive endoscopies. Given its classification as a Class B device, it is subject to stringent regulatory controls to ensure safety, effectiveness, and quality compliance before entering the Indian market.
Complying with CDSCO regulations is not merely a bureaucratic exercise—it is a critical step to ensure patient safety and market credibility. With the official notification 29/Misc./03/2020-DC (182) dated 27.09.2021, this device’s regulatory pathway has been clearly defined, facilitating smoother approvals.
CDSCO Regulatory Framework for Video Capsule Endoscopy Systems
The regulatory oversight for medical devices in India falls under the Central Drugs Standard Control Organization (CDSCO), which implements the Medical Device Rules, 2017. For a Class B device such as the Video Capsule Endoscopy System, the manufacturing license is granted by the State Licensing Authority through the MD5 license process.
These rules ensure that devices meet the Essential Principles of safety and performance, supported by robust documentation including Device Master File, Plant Master File, risk management, and quality management systems.
Risk Classification and License Requirements for the Video Capsule Endoscopy System
Class B devices are categorized as low to moderate risk requiring a Manufacturing License via Form MD3 (MD5 License). This license is issued by the State Licensing Authority after a thorough evaluation process that includes product testing and facility audit by a notified body.
For detailed classification and risk understanding, refer to our Medical Device Classification guide.
Manufacturing License Process (MD5) for Class B Devices
The MD5 license process for your Video Capsule Endoscopy System involves the following key steps:
- Test License Application (Form MD13): Start by obtaining a test license valid for 3 months. This allows sample testing at government-approved labs.
- Product Testing: Submit product samples to notified testing laboratories listed on the CDSCO Testing Laboratories portal.
- Documentation Preparation: Compile all required documents including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, and QMS documentation.
- Application Submission (Form MD3): Submit the manufacturing license application for MD5 through the CDSCO MD Online Portal.
- Audit by Notified Body: Coordinate with a notified body from the official list for a facility audit.
- Query Resolution: Address any observations or queries raised by the CDSCO or notified body promptly.
- License Grant (Form MD5): Upon satisfactory compliance, the license is granted.
For a detailed stepwise guide, you may find our MD5 License Guide very useful.
Manufacturing License Documents Required
To expedite your MD5 license application, ensure all of the following are meticulously prepared:
- Company Constitution Documents: Incorporation certificate, Memorandum and Articles of Association
- Proof of Premises Ownership or Lease Agreement: Valid documents verifying manufacturing site
- Technical Staff Qualifications: Details and experience of qualified personnel
- Fire NOC and Pollution Control NOC: From local authorities
- Device Master File (DMF): Detailed design and manufacturing process documentation (Guide here)
- Plant Master File (PMF): Manufacturing facility and quality control information (Guide here)
- Essential Principles Checklist: Compliance matrix with Indian regulations
- Risk Management File: Following ISO 14971 standards (Risk Management insights)
- Test Reports: From CDSCO-approved labs
- Labels and Instructions for Use (IFU): As per regulatory norms
- Quality Management System Documents: ISO 13485:2016 certification and related procedures
Preparing these documents comprehensively reduces delays and audit findings.
Import License Process (MD15) for Video Capsule Endoscopy Systems
If you intend to import the Video Capsule Endoscopy System instead of manufacturing locally, you must apply for the MD15 Import License. This process is governed by the Central Licensing Authority and typically takes 5-6 months.
Key steps include:
- Document Preparation: Manufacturing license from the country of origin, Free Sale Certificate, CE Certificate, Device and Plant Master Files, ISO 13485:2016 certificate, Wholesale License, and Company Constitution.
- Application Submission (Form MD14): Submit your application via the CDSCO MD Online Portal.
- Query Resolution: Respond promptly to any departmental queries.
- License Grant (Form MD15): Receive your import license upon compliance.
More details can be found in our Import License Guide.
Import License Documents Required
The import dossier must include:
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Quality Management System Certificate
- CE Certificate or equivalent
- Device Master File and Plant Master File
- Wholesale License in India
- Company Constitution documents
- Product labels and IFUs
Ensuring these are complete and valid expedites the review process.
Timeline and Processing Duration
| License Type | Processing Time |
|---|---|
| MD5 (Manufacturing) | 3-4 months total |
| Test License (MD13) | 1.5-2 months (initial) |
| Product Testing | Concurrent with test license |
| Audit & Query Resolution | Included in total timeline |
| MD15 (Import License) | 5-6 months |
Planning your project timeline with these durations in mind can prevent costly delays.
Government Fees and Costs
For a Class B device like the Video Capsule Endoscopy System, the fee structure for manufacturing license (MD5) is as follows:
- Application Fee: Rs 5,000 per application
- Product Fee: Rs 500 per product
Additional costs may include testing fees at government-approved laboratories and audit fees payable to notified bodies.
For import licenses (MD15), the fee depends on the class:
- Class B: Site Fee 1,000 per product
Budgeting appropriately for these fees upfront aids in smooth application processing.
Common Challenges and Solutions
Challenge: Delays due to incomplete documentation.
Solution: Use our detailed document checklists and templates to prepare your dossier thoroughly.
Challenge: Difficulty in coordinating audits with notified bodies.
Solution: Engage early with notified bodies listed on the CDSCO portal to schedule audits.
Challenge: Product testing bottlenecks.
Solution: Submit samples to CDSCO-approved labs promptly; consider parallel activities like document preparation during testing.
Challenge: Understanding regulatory updates.
Solution: Stay informed through official notifications and expert advisories.
Expert Consultation and Support
We have successfully guided over 500 medical device companies through the CDSCO licensing maze. Our expertise ensures:
- Precise classification and risk assessment
- Comprehensive and compliant documentation
- Efficient coordination with testing labs and notified bodies
- Timely query resolution and audit support
Partnering with experienced consultants can significantly reduce time-to-market and regulatory risks.
Getting Started with Your CDSCO License Application for Video Capsule Endoscopy Systems
- Assess your device classification and intended use carefully. Confirm Class B status under the Medical Device Classification guidelines.
- Initiate Test License application (Form MD13) promptly via the CDSCO MD Online Portal.
- Arrange product testing at CDSCO-approved laboratories to generate compliant test reports.
- Prepare your complete documentation, focusing on Device Master File, Plant Master File, and Risk Management files.
- Identify and liaise with a notified body early for your factory audit.
- Submit your MD5 license application (Form MD3) once test license and testing are complete.
- Track your application status regularly on the CDSCO portal and respond swiftly to any queries.
Embarking on this structured approach ensures your Video Capsule Endoscopy System meets all regulatory mandates and reaches the Indian market efficiently. For personalized assistance and a seamless licensing journey, connect with our regulatory experts today.