CDSCO License for Visual light box
Medical Device Information
Important Notice for Class A Devices
Class A Non-sterile Non Measuring devices are exempt from MD5 and MD15 licenses. Only CDSCO registration is required as per GSR 777(E).
Intended Use
A light viewing box that uses a translucent version of the ophthalmic chart (Snellen chart) used for testing visual acuity.
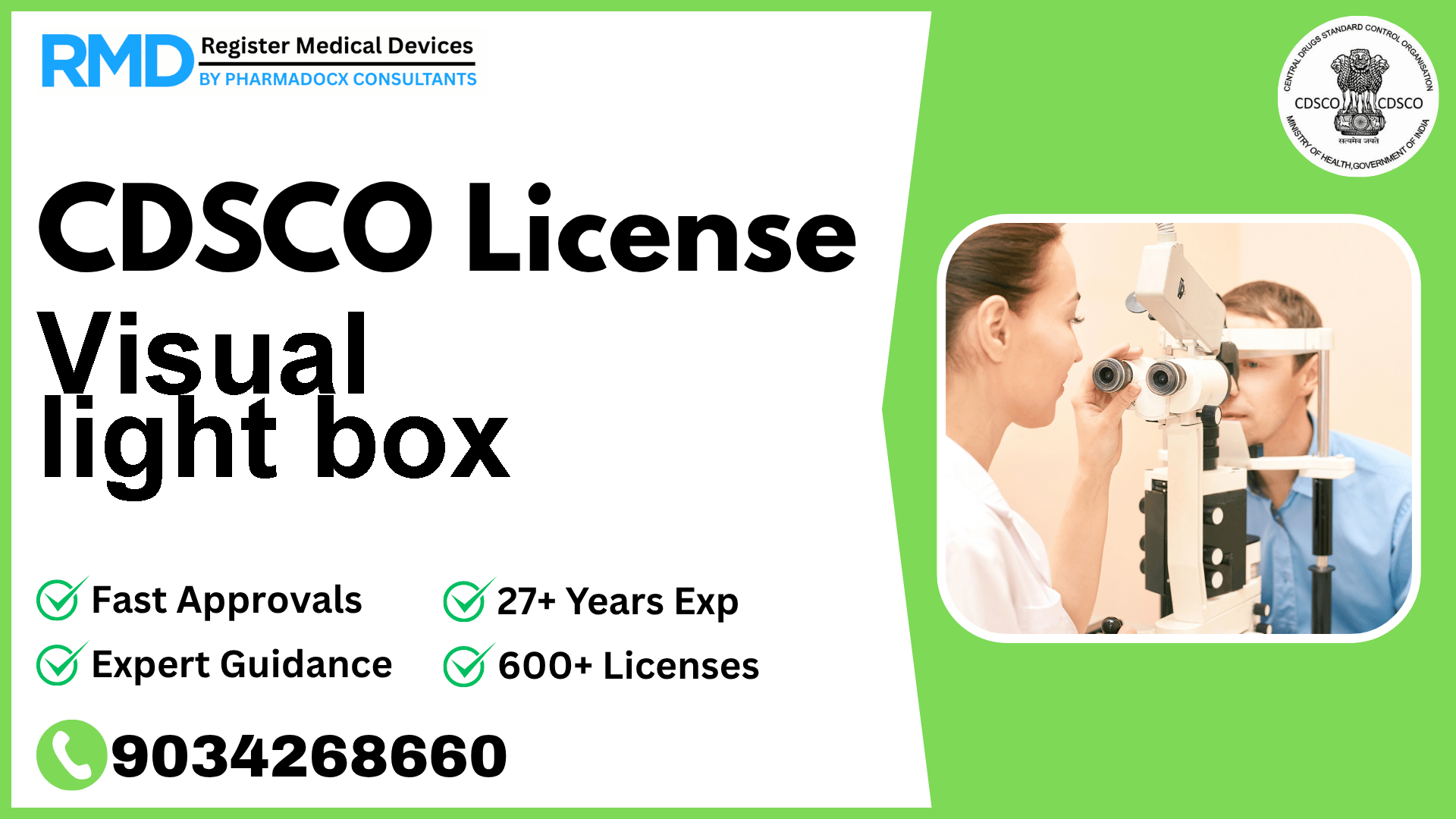
Comprehensive Guide to CDSCO Licensing for Visual Light Box (Class A Medical Device)
Visual light boxes, essential in ophthalmology for testing visual acuity using a translucent Snellen chart, fall under Class A medical devices in India. With the notification Fts No. 29/MiscJO3/2020-DC (187) dated 9.8.2021, regulatory compliance by the Central Drugs Standard Control Organization (CDSCO) is mandatory for manufacturers and importers aiming to market this device in India.
Having supported over 500 companies in securing CDSCO licenses, we provide you with a detailed, actionable roadmap to navigate the licensing process efficiently, focusing on the unique requirements for your visual light box.
CDSCO Regulatory Framework for Visual Light Box
The CDSCO governs medical devices under the Medical Device Rules, 2017, classifying devices based on risk. As a Class A device, the visual light box is considered low risk but still requires formal manufacturing or import licensing. Compliance ensures product safety, quality, and legal market authorization.
Manufacturers must secure the MD5 manufacturing license from the State Licensing Authority, while importers require the MD15 import license from the Central Licensing Authority.
Risk Classification and License Requirements
Your visual light box is categorized as a Class A medical device per the CDSCO classification, meaning:
- Risk Level: Low
- License Type: MD5 (Manufacturing License) for domestic manufacturers
- Regulatory Authority: State Licensing Authority
- Notification Reference: Fts No. 29/MiscJO3/2020-DC (187), dated 9.8.2021
For detailed classification insights, refer to our Medical Device Classification guide.
Manufacturing License Process (MD5) for Visual Light Box
The MD5 license process is multi-stage and takes approximately 3 to 4 months, structured as follows:
- Test License (Form MD13) Application: Initiate by applying for a Test License on the CDSCO MD Online Portal, which takes about 1.5 to 2 months to process.
- Product Testing: Get your visual light box tested at government-approved laboratories. Refer to the official list of testing laboratories.
- Document Preparation: Prepare comprehensive documentation, including Device Master File, Plant Master File, and essential compliance files.
- Application for Manufacturing License (Form MD3): Submit your MD5 application on the CDSCO MD Online Portal.
- Audit by Notified Body: A notified body will audit your manufacturing facility. Check the list of notified bodies for authorized auditors.
- Query Resolution: Respond promptly and comprehensively to any queries from the licensing authority or notified body.
- Grant of License (Form MD5): Upon satisfactory audit and documentation review, the license is granted.
For a deep dive into the MD5 licensing process, our MD5 License Guide is an invaluable resource.
Manufacturing License Documents Required for Visual Light Box
To expedite your MD5 application, ensure you have the following documents meticulously prepared:
- Company Constitution/Registration Certificate
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Technical Staff Qualification and Experience Details
- Fire NOC and Pollution Control Board NOC
- Device Master File (DMF): Detailed device specifications, manufacturing process, and quality controls. Learn more from our Device Master File guide.
- Plant Master File (PMF): Details about the manufacturing facility and equipment. See our Plant Master File guide.
- Essential Principles Checklist: Demonstrate compliance with safety and performance requirements.
- Risk Management File: Documentation of identified risks and mitigation strategies. For best practices, visit our Risk Management guide.
- Test Reports: From CDSCO-approved testing laboratories.
- Labels and Instructions for Use (IFU): Clear and compliant.
- Quality Management System (QMS) Documents: Including ISO 13485 certification evidence.
Import License Process (MD15) for Visual Light Box
If you are importing visual light boxes, the MD15 import license is mandatory. The process involves:
- Document Preparation: Compile import-specific documents, including your manufacturing license from the country of origin.
- Application Submission: Apply on the CDSCO MD Online Portal using Form MD14.
- Query Resolution: Address any department queries promptly.
- License Grant: The process usually takes 5 to 6 months.
For a thorough explanation, explore our Import License Guide.
Import License Documents Required
- Manufacturing License of the foreign manufacturer
- Free Sale Certificate from the country of origin
- ISO 13485:2016 Certification
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License in India
- Company Constitution
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 2 – 3 weeks |
| Document Preparation | 2 – 3 weeks |
| Manufacturing License Application (MD5) | 1 – 2 weeks for submission |
| Audit by Notified Body | 3 – 4 weeks |
| Query Resolution | 2 – 4 weeks |
| Total Estimated Time | 3 – 4 months |
Proactive documentation and rapid query response can significantly shorten these timelines.
Government Fees and Costs
For your Class A visual light box, the fee structure is as follows:
- MD5 License Application Fee: Rs. 5,000 per application
- Per Product Fee: Rs. 500 per product
Additional costs to budget for include testing fees at government-approved labs and notified body audit charges, which vary depending on the auditing agency.
Common Challenges and Solutions
Challenge: Delays in Test License Approval
Solution: Submit complete and accurate test license applications with all required documents, and maintain close communication with the licensing authority.
Challenge: Incomplete or Non-Compliant Documentation
Solution: Use checklists and seek expert review of your Device and Plant Master Files, Risk Management files, and QMS documents before submission.
Challenge: Audit Non-Conformities
Solution: Conduct internal audits and pre-audit assessments to identify and rectify potential issues before the notified body visits.
Challenge: Query Resolution Delays
Solution: Assign dedicated personnel to monitor communications and prepare prompt, detailed responses.
Expert Consultation and Support
With over 25 years of experience and a proven track record of assisting over 500 companies, we offer tailored consultancy to:
- Prepare all necessary documentation
- Coordinate with notified bodies and testing labs
- Manage application submissions via the CDSCO MD Online Portal
- Navigate audit processes efficiently
Our expertise ensures your visual light box moves seamlessly from development to market launch.
Getting Started with Your CDSCO License Application
- Assess Your Product Classification: Confirm your visual light box’s Class A status with CDSCO.
- Initiate Test License Application: Submit Form MD13 through the CDSCO MD Online Portal.
- Prepare Device and Plant Master Files: Utilize our guides to compile comprehensive documentation.
- Engage a Notified Body Early: Select an auditor from the official Notified Bodies List to schedule your facility audit.
- Plan for Testing: Identify a government-approved laboratory to conduct product tests.
- Compile Complete Application: Gather all required documentation as per the checklist.
- Submit Your MD5 Application: After successful test license and testing, apply on the CDSCO portal.
- Prepare for Audit and Queries: Conduct internal reviews and be ready to provide clarifications.
By following this structured approach and leveraging expert support, your path to obtaining the CDSCO license for your visual light box will be efficient and compliant.
For personalized assistance, contact us to leverage our deep regulatory knowledge and hands-on experience in medical device licensing across India.