CDSCO License for Anaesthetic Conduction Kit
Medical Device Information
Intended Use
An anaesthesia conduction kit is a device used to administer to a patient conduction, regional, or local anaesthesia. The device may contain syringes, needles, and drugs.
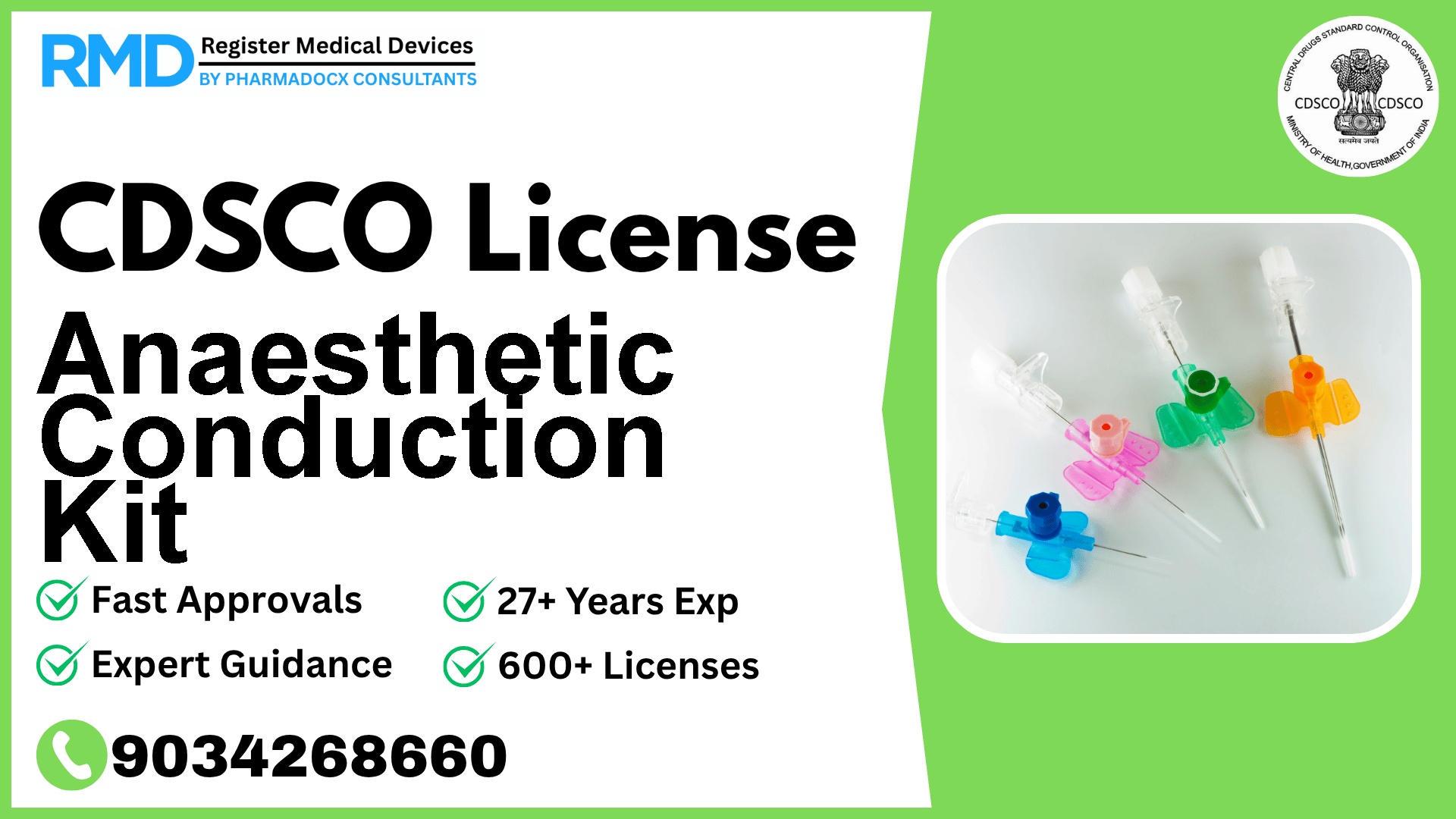
Comprehensive Guide to CDSCO Licensing for Anaesthetic Conduction Kit (Class C Medical Device)
As seasoned regulatory consultants with over 25 years of experience and having supported 500+ companies in obtaining CDSCO licenses, we understand the complexities involved in registering and licensing medical devices in India. This guide focuses specifically on the Anaesthetic Conduction Kit, a Class C medical device under the category of Catheters, intended for administering regional, conduction, or local anesthesia. Navigating the CDSCO regulatory framework efficiently is critical to accessing the Indian market and ensuring compliance.
Understanding the Anaesthetic Conduction Kit and Its Regulatory Importance
An Anaesthetic Conduction Kit typically includes syringes, needles, and drugs designed to facilitate safe and effective anesthesia administration. Given its direct interaction with patients and potential risks, the device falls under Class C, indicating a moderate to high risk. Proper regulatory approval ensures the device meets safety, efficacy, and quality standards mandated by the Central Drugs Standard Control Organization (CDSCO).
CDSCO Regulatory Framework for Anaesthetic Conduction Kit
The CDSCO classifies medical devices into four classes (A, B, C, D) based on risk. The Anaesthetic Conduction Kit is classified as Class C under Notification 29/Misc/3/2017-DC (292) dated 06.06.2018. For Class C devices, the manufacturing license is issued by the Central Licensing Authority in the form of an MD9 license, governed by the Drugs and Cosmetics Act and Rules.
Risk Classification and License Requirements for Class C Devices
Class C devices require stringent regulatory control due to their risk profile. The MD9 license is mandatory for manufacturing such devices in India. This license confirms compliance with technical, quality, and safety standards, including essential principles, risk management, and post-market surveillance.
Manufacturing License Process for Anaesthetic Conduction Kit (MD9 License)
The manufacturing license for Class C Anaesthetic Conduction Kit involves a detailed multi-step process:
- Test License (Form MD13): Initially, a test license must be obtained to manufacture and test the device. This takes approximately 1.5 to 2 months.
- Product Testing: Samples must be tested at CDSCO-approved testing laboratories to verify compliance. Check the list of testing laboratories for approved facilities.
- Document Preparation: Comprehensive documentation including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, and Quality Management System (QMS) documents must be prepared.
- Application Submission (Form MD7): Submit the manufacturing license application on the CDSCO MD Online Portal along with the required fees.
- Inspection and Audit: CDSCO inspectors conduct a thorough audit of manufacturing premises, processes, and documentation.
- Queries Resolution: Address any queries raised by the department or inspectors promptly.
- License Grant: Upon successful inspection and document approval, the MD9 license is granted.
For a detailed guide on the MD9 license process, you can refer to our MD9 License Guide.
Manufacturing License Documents Required for Anaesthetic Conduction Kit
To ensure a smooth application process, prepare the following key documents:
- Company Constitution or Incorporation Certificate
- Proof of Ownership or Lease Agreement of Manufacturing Premises
- Details and Qualifications of Technical Staff
- Fire No Objection Certificate (NOC)
- Pollution Control Board NOC
- Device Master File (DMF) detailing design, manufacturing, and safety aspects (Device Master File Guide)
- Plant Master File (PMF) describing manufacturing processes and quality controls (Plant Master File Guide)
- Essential Principles Checklist demonstrating compliance with Indian regulatory requirements
- Risk Management File outlining hazard analysis and mitigation (Risk Management)
- Test Reports from CDSCO-approved labs
- Product Labels and Instructions for Use (IFU)
- Quality Management System documentation (ISO 13485:2016 certification recommended)
Import License Process for Anaesthetic Conduction Kit (MD15 License)
For importers, the MD15 import license is mandatory, issued by the Central Licensing Authority. The steps include:
- Document Preparation: Gather documents such as the manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016 certificate, CE Certificate, Device and Plant Master Files, wholesale license, and company constitution.
- Application Submission: File the application on the CDSCO MD Online Portal using Form MD14.
- Queries Resolution: Engage with CDSCO officials to resolve any clarifications.
- License Grant: Upon acceptance, the MD15 import license is granted.
Refer to our comprehensive Import License Guide for step-by-step assistance.
Import License Documents Required
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate or equivalent international certifications
- Device Master File
- Plant Master File
- Wholesale License for import
- Company Constitution and Incorporation documents
Timeline and Processing Duration
Manufacturing License (MD9):
- Test License (MD13): 1.5 to 2 months
- Product Testing: 1 to 1.5 months
- Application Processing and Audit: 1.5 to 2 months
- Total Estimated Time: Approximately 4 to 5 months
Import License (MD15):
- Application Review and Approval: 5 to 6 months
Government Fees and Costs
MD9 Manufacturing License:
- Application Fee: ₹50,000 per application
- Product Fee: ₹1,000 per product
MD15 Import License:
- Class C Device Fees: $3,000 per site
- Product Fee: $1,500 per product
These fees are paid via the CDSCO online portal during application submission.
Common Challenges and Practical Solutions
- Delayed Testing Reports: To avoid bottlenecks, pre-select testing labs from the official CDSCO testing laboratories list and schedule testing well in advance.
- Incomplete Documentation: Use checklists and templates aligned with CDSCO’s requirements. Our guides on Device Master File and Plant Master File can streamline document preparation.
- Audit Non-compliance: Conduct internal mock audits and gap assessments before official inspections to ensure readiness.
- Query Resolution Delays: Assign dedicated personnel to monitor CDSCO communications and respond swiftly to queries.
Expert Consultation and Support
Navigating CDSCO’s regulatory landscape for Class C devices like Anaesthetic Conduction Kits requires expertise. Our team offers end-to-end consultancy services including document preparation, audit support, product testing coordination, and liaison with CDSCO authorities to expedite approvals.
Getting Started with Your CDSCO License Application
- Assess Device Classification: Confirm your device is Class C under the notified CDSCO classification (Medical Device Classification).
- Initiate Test License (MD13) Application: Prepare and submit your test license application to begin manufacturing and testing.
- Engage Approved Testing Labs: Coordinate with notified labs for timely testing of your Anaesthetic Conduction Kit.
- Prepare Complete Documentation: Assemble all required files, using our Device and Plant Master File guides for accuracy.
- Submit MD9 License Application: Once test license and testing are complete, submit the MD9 manufacturing license application on the CDSCO MD Online Portal.
- Prepare for Inspection: Conduct internal audits and finalize compliance before CDSCO inspection.
- Resolve Queries Promptly: Maintain open communication with CDSCO to address any issues quickly.
Embarking on this process with clear guidance and expert support will significantly increase your chances of securing the MD9 manufacturing license for your Anaesthetic Conduction Kit, enabling you to confidently enter the Indian medical device market.
For personalized assistance tailored to your product and company specifics, contact us today and leverage our 25+ years of expertise in CDSCO licensing.