CDSCO License for Anesthesia conduction filter
Medical Device Information
Intended Use
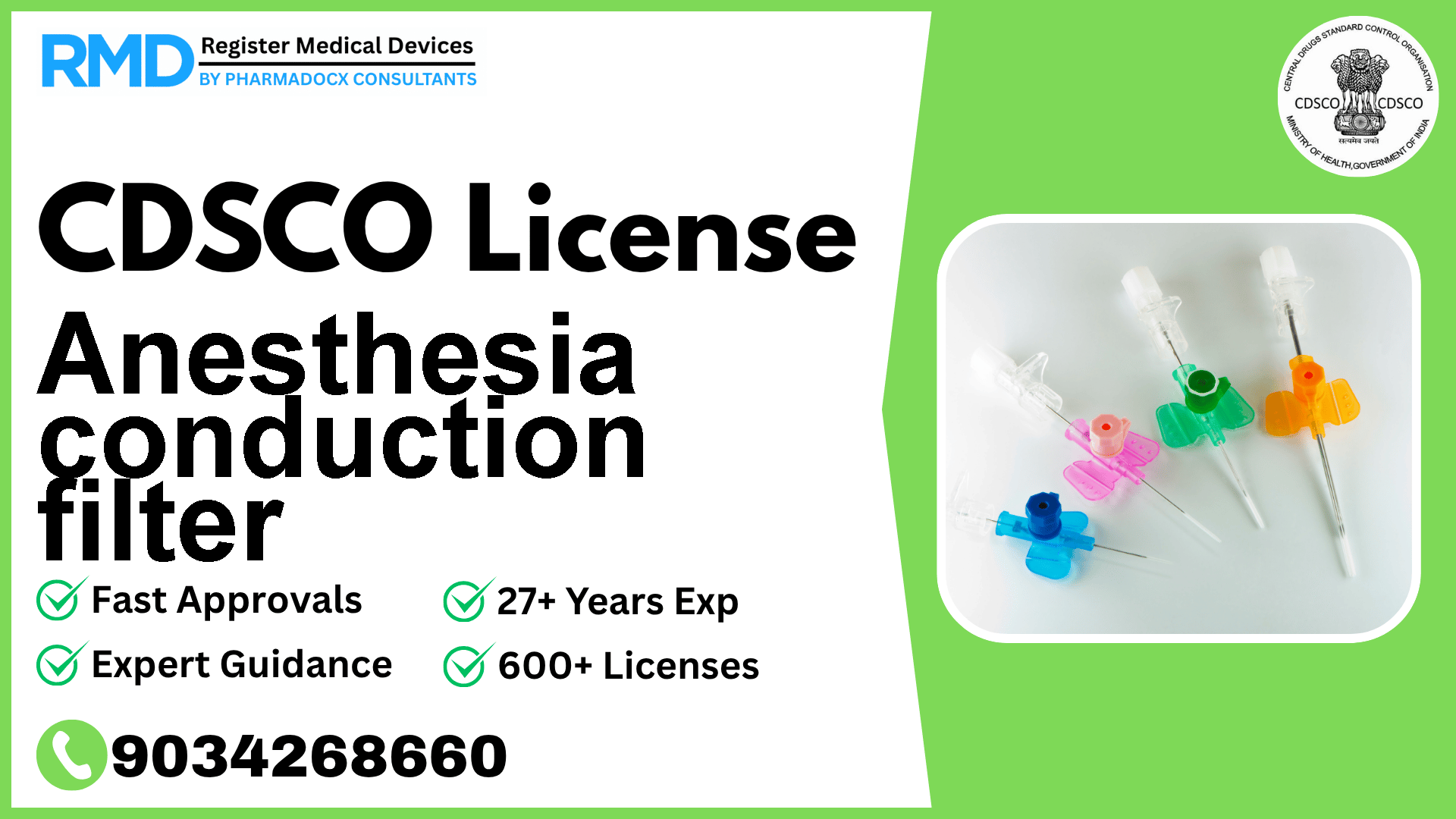
A microporous filter used while administering to a patient injections of local anesthetics to minimize particulate (foreign material) contamination of the injected fluid
Comprehensive Guide to CDSCO Licensing for Anesthesia Conduction Filter (Class C Catheter)
Anesthesia conduction filters are critical microporous devices used during the administration of local anesthetics to minimize particulate contamination in injected fluids. Classified under Catheters, these filters fall under Class C risk category as per CDSCO notification [29/Misc/3/2017-DC (292)] dated 06.06.2018. Navigating the regulatory landscape for such devices in India can be complex, especially given their high-risk classification. With over 25 years of experience assisting 500+ medical device companies, we provide an authoritative, step-by-step guide tailored for manufacturers and importers of Anesthesia Conduction Filters.
CDSCO Regulatory Framework for Anesthesia Conduction Filters
The Central Drugs Standard Control Organization (CDSCO) governs medical device licensing in India under the Medical Device Rules (MDR) 2017. Anesthesia conduction filters, being Class C devices, require stringent compliance to ensure patient safety. Regulatory oversight involves:
- Central Licensing Authority (CLA) approval for manufacturing (MD9 license)
- CLA approval for import (MD15 license)
- Compliance with Essential Principles of Safety and Performance
- Documentation of risk management, quality systems, and testing
The regulatory framework ensures that devices meet both Indian and international standards, enabling smooth market entry and sustained compliance.
Risk Classification and License Requirements for Class C Devices
Class C medical devices, such as Anesthesia Conduction Filters, are considered moderate-to-high risk. Consequently, manufacturers must obtain a MD9 Manufacturing License issued by the Central Licensing Authority. Importers must secure an MD15 Import License from the same authority.
License Summary for Class C Anesthesia Conduction Filter:
| License Type | Authority | Timeline | Fees | Application Form |
|---|---|---|---|---|
| Manufacturing (MD9) | Central Licensing Authority | 4-5 months | ₹50,000 + ₹1,000 per product | MD7 |
| Import (MD15) | Central Licensing Authority | 5-6 months | Variable (₹2,20,000 approx.) | MD14 |
For detailed device classification, refer to our Medical Device Classification guide.
Manufacturing License Process (MD9 License) for Anesthesia Conduction Filters
The MD9 license process is multi-phased and requires meticulous preparation:
Test License (Form MD13): Before applying for MD9, obtain a test license allowing limited manufacture for product testing. This takes approximately 1.5 to 2 months.
Product Testing: Submit samples to government-approved labs for mandatory testing. Testing labs can be found in the Testing Laboratories list.
Documentation Preparation: Compile extensive documentation including Device Master File, Plant Master File, Essential Principles Checklist, Risk Management File, and QMS documents.
Application Submission: Apply on the CDSCO MD Online Portal using Form MD7.
Audit by CDSCO Inspectors: The licensing authority conducts a thorough audit of the manufacturing site and quality systems.
Query Resolution: Address any queries raised by CDSCO or the audit team promptly.
License Grant: Upon satisfactory compliance, the MD9 license is granted.
For a detailed walkthrough, see our MD9 License Guide.
Manufacturing License Documents Required for MD9
Accurate and complete documentation is critical. Required documents include:
- Company Constitution (Incorporation Certificate)
- Proof of Premises Ownership or Lease
- Technical Staff Qualification and Experience Certificates
- Fire and Pollution NOCs
- Device Master File (DMF) detailing design, manufacturing processes, and specifications (Device Master File Guide)
- Plant Master File (Plant Master File Guide)
- Essential Principles Checklist demonstrating compliance with MDR 2017
- Risk Management File per ISO 14971 (Risk Management)
- Test Reports from CDSCO-approved laboratories
- Labeling and Instructions For Use (IFU)
- Quality Management System (QMS) documentation, preferably ISO 13485:2016 certified
Import License Process (MD15 License) for Anesthesia Conduction Filters
Importers seeking to bring Anesthesia Conduction Filters into India must obtain an MD15 license from CDSCO’s Central Licensing Authority. The process includes:
Document Preparation: Obtain manufacturing license from the country of origin, Free Sale Certificate, ISO 13485:2016 certification, CE Certificate (if applicable), and CDSCO-required technical files.
Application Submission: File application via Form MD14 on the CDSCO MD Online Portal.
Query Resolution: Respond promptly to any clarifications sought by CDSCO.
License Issuance: MD15 license is granted, typically within 5-6 months.
For detailed steps, see our Import License Guide.
Import License Documents Required for MD15
- Valid Manufacturing License from the country of origin
- Free Sale Certificate
- ISO 13485:2016 Certificate
- CE Certificate (if applicable)
- Device Master File and Plant Master File
- Wholesale License (if applicable)
- Company Constitution
- Labeling and IFU
Timeline and Processing Duration
| Process Step | Duration |
|---|---|
| Test License (MD13) | 1.5 – 2 months |
| Product Testing | 1 – 1.5 months |
| Documentation Preparation | 1 month |
| MD9 License Application | 4 – 5 months |
| MD15 Import License | 5 – 6 months |
Overall, expect approximately 4-5 months for manufacturing license and 5-6 months for import license, contingent on timely responses and audit outcomes.
Government Fees and Costs
MD9 Manufacturing License Fees:
- Application Fee: ₹50,000
- Per Product Fee: ₹1,000
MD15 Import License Fees (Approximate):
- Class C Device Fees: ₹3,00,000 per site + ₹1,50,000 per product (converted to INR approx.)
Note that fees are subject to change per CDSCO norms. Additional costs include audit fees, testing charges at government notified labs, and consultancy fees if you opt for expert assistance.
Common Challenges and Practical Solutions
Challenge 1: Delays in obtaining test licenses and scheduling lab testing.
Solution: Initiate the test license application early and coordinate with approved labs beforehand to ensure sample acceptance and timely testing.
Challenge 2: Incomplete or inconsistent documentation leading to queries.
Solution: Use detailed checklists and templates for Device Master File, Risk Management, and QMS. Regularly update documents to reflect current processes.
Challenge 3: Non-compliance during CDSCO audits.
Solution: Conduct internal audits and mock inspections prior to CDSCO visits. Engage with notified bodies listed in the Notified Bodies List for expert audit guidance.
Challenge 4: Complex import documentation requirements.
Solution: Maintain a comprehensive Import Dossier and ensure all international certifications are valid and translated into English if needed.
Expert Consultation and Support
Navigating the CDSCO regulatory pathway for Class C devices like Anesthesia Conduction Filters demands specialized expertise. Our seasoned consultants bring over two decades of in-depth knowledge and have successfully guided more than 500 companies through the entire licensing journey. From document preparation to audit readiness and query management, we provide end-to-end support ensuring timely approval and compliance.
Getting Started with Your CDSCO License Application
Assess Your Device Classification: Confirm Class C status using official CDSCO guidelines.
Register on the CDSCO MD Online Portal: Create your account at CDSCO MD Online Portal.
Gather Preliminary Documents: Company incorporation, premises proof, and technical staff records.
Apply for Test License (MD13): Initiate test license application to start product testing.
Coordinate Product Testing: Engage with notified government labs early to schedule testing.
Prepare Complete Documentation: Device Master File, Plant Master File, Risk Management, QMS, labels, and IFU.
Submit Manufacturing License Application (MD7): After successful testing, file for MD9 license.
Prepare for Audit: Conduct internal audit and ensure compliance before CDSCO inspection.
Address Queries Promptly: Respond comprehensively to any CDSCO or auditor queries.
Plan for Import License: If importing, concurrently prepare for MD15 license application.
By following this roadmap, you can significantly reduce delays and increase your chances of swift regulatory approval for your Anesthesia Conduction Filter in the Indian market.
For personalized assistance and detailed regulatory strategy, contact our expert team today and leverage our 25+ years of CDSCO licensing excellence.