CDSCO License for Balloon Dialation Vessel Catheter
Medical Device Information
Intended Use
Intended for use in Percutaneous Transluminal Angioplasty of the renal, tibial, popliteal, femoral and peroneal arteries. These catheters are not for use in coronary arteries.
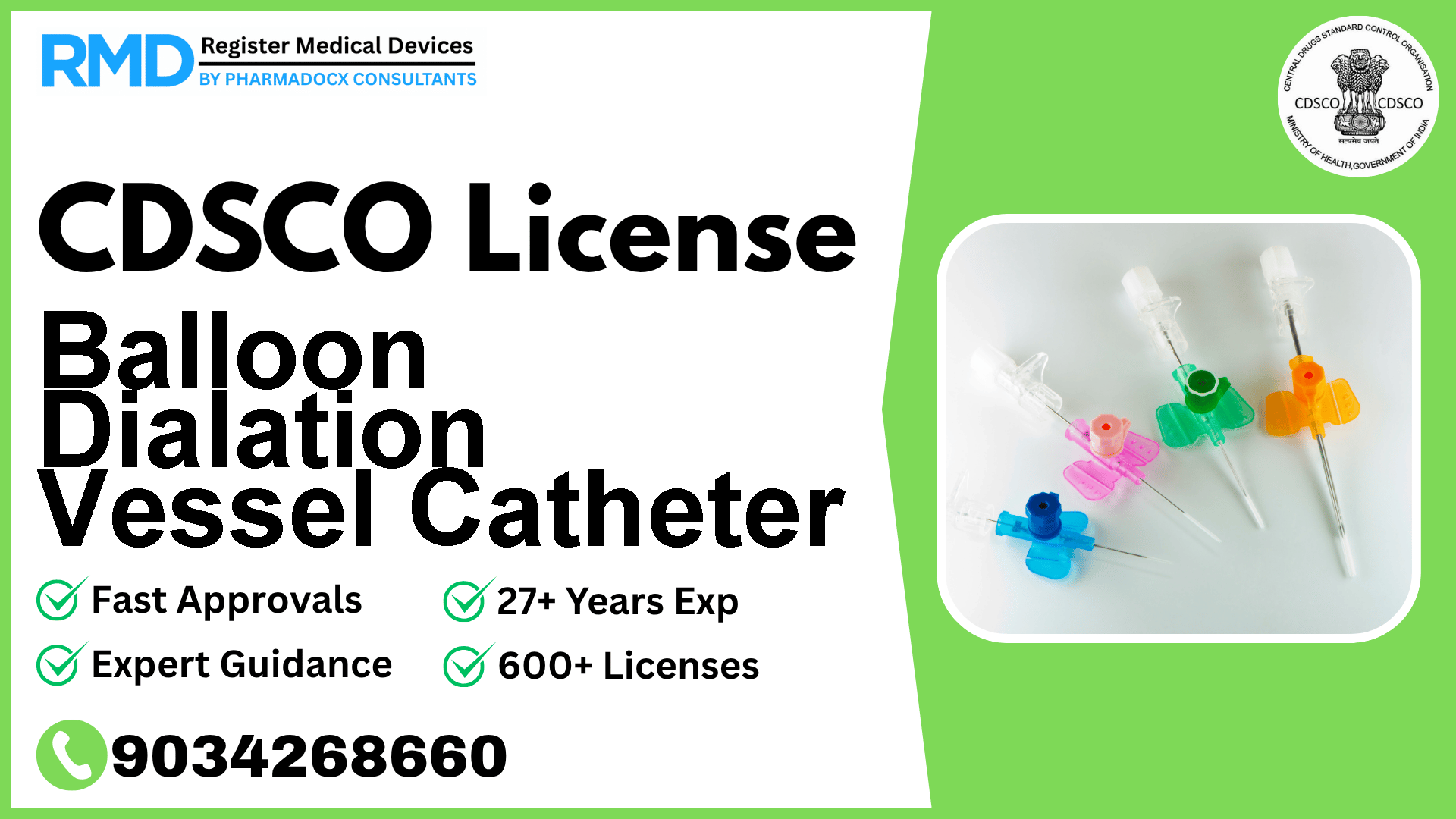
Introduction to Balloon Dilation Vessel Catheter and Regulatory Importance
Balloon Dilation Vessel Catheters are critical medical devices used in percutaneous transluminal angioplasty to treat blockages in peripheral arteries such as renal, tibial, popliteal, femoral, and peroneal arteries. Not intended for coronary artery use, these catheters fall under Class B risk category as per CDSCO regulations, reflecting a moderate risk profile requiring stringent regulatory oversight.
Given their vital role in vascular interventions, ensuring compliance with the Central Drugs Standard Control Organization (CDSCO) licensing requirements in India is crucial. Compliance not only guarantees patient safety but also facilitates market access and builds trust among healthcare providers.
CDSCO Regulatory Framework for Balloon Dilation Vessel Catheters
Balloon Dilation Vessel Catheters are classified under catheters category and regulated under the Medical Device Rules (MDR), 2017 notified by CDSCO. The specific notification applicable is 29/Misc/3/2017-DC (292) dated 06.06.2018, which governs their manufacture and import into the Indian market.
The CDSCO framework mandates obtaining appropriate licenses based on risk classification, with Class B devices requiring an MD5 manufacturing license issued by the State Licensing Authority.
Risk Classification and License Requirements
As a Class B medical device, the Balloon Dilation Vessel Catheter is considered moderate risk. This classification necessitates obtaining the MD5 manufacturing license for local production or MD15 import license if the device is imported into India.
- Class B Device: MD5 License (Manufacturing) via Form MD3
- Import License: MD15 License via Form MD14
The MD5 license process includes product testing, audit by notified bodies, and submission of comprehensive documentation.
Manufacturing License Process (MD5) for Balloon Dilation Vessel Catheters
For manufacturers planning to produce Balloon Dilation Vessel Catheters in India, the MD5 license process is mandatory. The process generally spans 3 to 4 months and involves the following key steps:
- Test License Application (Form MD13): Obtain a test license enabling sample testing, which takes approximately 1.5 to 2 months.
- Product Testing: Conduct mandatory product testing at CDSCO-recognized laboratories. Testing covers biocompatibility, sterility, dimensional accuracy, and performance parameters.
- Document Preparation: Assemble the required documentation including Device Master File (DMF), Plant Master File (PMF), Risk Management File, and Quality Management System (QMS) documents.
- Application for MD5 License (Form MD3): Submit your application through the CDSCO MD Online Portal along with fees.
- Audit by Notified Body: Engage with a notified body from the list of notified bodies for a thorough audit of manufacturing facilities and processes.
- Queries and Clarifications: Address any queries raised by the regulatory authority or notified body promptly.
- Grant of MD5 License: Upon satisfactory review, the license is granted on Form MD5.
Manufacturing License Documents Required
Successful MD5 license applications for Balloon Dilation Vessel Catheters must include:
- Company Constitution and Registration Documents
- Proof of Ownership or Lease of Manufacturing Premises
- Details and Qualification Certificates of Technical Staff
- Fire and Pollution NOCs
- Device Master File (DMF) detailing device specifications and design (read our DMF guide)
- Plant Master File (PMF) describing manufacturing processes (PMF guide)
- Essential Principles Checklist demonstrating compliance with Indian MDR
- Risk Management File evidencing hazard analysis and mitigation (risk management best practices)
- Product Test Reports from CDSCO-approved laboratories (testing labs list)
- Product Labels and Instructions for Use (IFU)
- Quality Management System documentation (ISO 13485 compliant)
Import License Process (MD15) for Balloon Dilation Vessel Catheters
For importers seeking to bring Balloon Dilation Vessel Catheters into India, an MD15 import license is mandatory. This license is granted by the Central Licensing Authority and usually takes 5 to 6 months to process.
Key steps include:
- Document Preparation: Compile manufacturing license of the foreign manufacturer, Free Sale Certificate, CE Certificate (if applicable), DMF, PMF, Wholesale License, and Company Constitution.
- Application Submission (Form MD14): Submit the application via the CDSCO MD Online Portal.
- Review and Queries: Respond to any departmental queries promptly.
- Grant of MD15 License: Upon clearance, the import license is issued.
Unlike manufacturing license, no test license is required for imports.
Import License Documents Required
The MD15 application must include:
- Valid Manufacturing License of the foreign manufacturer
- Free Sale Certificate from country of origin
- ISO 13485:2016 Certificate
- CE Certificate or equivalent regulatory approval
- Device Master File and Plant Master File
- Wholesale Drug License
- Company Constitution and Address Proof
Timeline and Processing Duration
| License Type | Processing Time | Key Milestones |
|---|---|---|
| MD5 Manufacturing | 3-4 months | Test license (1.5-2 months), testing, audit, license issuance |
| MD15 Import | 5-6 months | Document review, queries, license issuance |
Timely submission of complete documents and prompt responses to queries can significantly reduce processing delays.
Government Fees and Costs
For Balloon Dilation Vessel Catheters (Class B), the official fees are:
- MD5 License: Rs. 5,000 per application + Rs. 500 per product
- MD15 Import License: 1,000 per product
Additional costs include testing fees at government approved labs, notified body audit charges, and consultancy fees if applicable.
Common Challenges and Solutions
Challenge 1: Delays due to incomplete documentation
- Solution: Use comprehensive checklists and consult expert regulatory consultants to ensure all documents meet CDSCO requirements.
Challenge 2: Product testing failures or repeated queries
- Solution: Pre-validate samples internally and select reputed CDSCO-approved labs to reduce test failures.
Challenge 3: Audit non-compliance
- Solution: Conduct internal audits and gap analysis before notified body inspection.
Challenge 4: Understanding complex MDR requirements
- Solution: Engage with experienced consultants who have supported 500+ companies in successful licensing.
Expert Consultation and Support
Navigating the CDSCO licensing landscape can be challenging, especially for specialized devices like Balloon Dilation Vessel Catheters. With over 25 years of experience and a proven track record assisting 500+ manufacturers and importers, we offer tailored regulatory support:
- Gap analysis and document preparation
- Coordination with notified bodies and testing labs
- Application filing and follow-up
- Training on MDR compliance and risk management
Our expertise ensures smoother approvals and faster time to market.
Getting Started with Your CDSCO License Application
- Assess Device Classification: Confirm your Balloon Dilation Vessel Catheter is Class B under MDR 2017.
- Initiate Test License (for Manufacturing): Apply for Form MD13 via CDSCO MD Online Portal.
- Engage Testing Labs and Begin Testing: Select from CDSCO-approved labs to conduct product tests.
- Prepare Complete Documentation: Compile DMF, PMF, Risk Management File, QMS documents, and other mandatory paperwork.
- Select a Notified Body for Audit: Refer to the list of notified bodies and schedule audits.
- Submit MD5 License Application (Form MD3): Upload your application and pay fees through the CDSCO portal.
- Monitor and Respond to Queries: Ensure timely communication with CDSCO and notified bodies.
For importers, begin with document compilation and apply for MD15 license using Form MD14.
Embarking on the licensing journey with a clear roadmap and expert guidance ensures your Balloon Dilation Vessel Catheter meets all regulatory standards, accelerating your entry into the Indian medical device market.